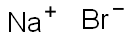
Sodium bromide synthesis
- Product Name:Sodium bromide
- CAS Number:7647-15-6
- Molecular formula:NaBr
- Molecular Weight:102.89
Sodium bromide can be made by passing bromine through an aqueous solution of sodium hydroxide or carbonate in the presence of a reducing agent, such as ammonia, hydrazine, activated charcoal, or Fe2+ ion. A typical method involves adding iron to bromine water to form ferrosoferric bromide, Fe[FeBr5]. This double salt is dissolved in excess water followed by addition of sodium carbonate. The product mixture is filtered and the filtrate is evaporated to crystallize sodium bromide. The overall reaction may be written as follows: 3Fe + 4Br2 + 4Na2CO3 → 8NaBr + FeCO3 + Fe2(CO3)3
Another method involves adding excess bromine to a solution of sodium hydroxide. This forms sodium bromide and bromate. The product solution is evapoated to dryness. The bromate is reduced to bromide by heating with carbon: 3Br2 + 2NaOH + H2O → NaBr + NaBrO3 + 4HBr.
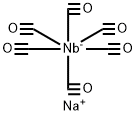
15602-39-8
0 suppliers
inquiry
18820-82-1
326 suppliers
$5.00/10g
![Niobium, bromotetracarbonyl[1,2-ethanediylbis[diphenylphosphine]-P,P']-, (TPS-7-1-233332)- (9CI)](/CAS/20211123/GIF/112713-60-7.gif)
112713-60-7
0 suppliers
inquiry
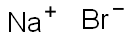
7647-15-6
873 suppliers
$12.50/100G
Yield:112713-60-7 78%
Reaction Conditions:
with C2H4(P(C6H5)2)2 in tetrahydrofuran;toluene;byproducts: C5H5N, CO, H2; Treating of suspn. of diphos and (H-py)Br in THF with Nb-complex at room temp. under a CO atmosphere.; Immediate react. with evolution of CO and H2 (gas chromy.) obsd. Stirring (2 h, room temp.), removal of THF (vac., room temp.), dissolving of residue (toluene, CO), stirring (2 h), filtn., treating with n-heptane. Sepn. of microcryst., elem. anal.;
References:
Calderazzo, Fausto;Pampaloni, Guido;Pelizzi, Giancarlo;Vitali, Francesca [Organometallics,1988,vol. 7,# 5,p. 1083 - 1092]
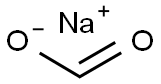
141-53-7
746 suppliers
$6.00/100g
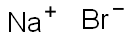
7647-15-6
873 suppliers
$12.50/100G