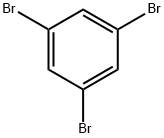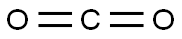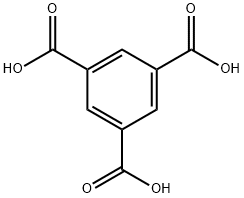
Trimesic acid synthesis
- Product Name:Trimesic acid
- CAS Number:554-95-0
- Molecular formula:C9H6O6
- Molecular Weight:210.14
Yield:554-95-0 97%
Reaction Conditions:
Stage #1: carbon dioxidewith o-phenylenebis(diphenylphosphine);copper(II) acetate monohydrate in 1,4-dioxane at 65; for 0.416667 h;Schlenk technique;
Stage #2: 1,3,5-trisbromobenzenewith palladium diacetate;triethylamine;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane;toluene at 100; for 16 h;Schlenk technique;
Steps:
23 Preparation of m-dibenzoic acid
General procedure: In a Schlenk (50 mL) reaction flask with a carbon dioxide balloon attached to a branch tube, add Cu (OAc) 2.H2O (0.02 mmol), 1,2-bis (diphenylphosphine) benzene (0.033 mmol), and 1,4 -Dioxane (3.0mL), PMHS (4.0mmol) was added under stirring, and then the reaction flask was placed in an oil bath at 65 ° C for 25min to react under a carbon dioxide atmosphere, followed by toluene (15mL), 3- Bromoiodobenzene (1.0mmol), Pd (OAc) 2 (0.05mmol), Xantphos (0.06mmol), Et3N (5.0mmol, 5.0equiva.), Then remove the carbon dioxide and react at 100 ° C until the raw bromobenzene disappears. After cooling to room temperature, a sodium hydroxide solution (1M, 20.0mL) was added, and the mixture was stirred for 10 minutes and filtered. The solid was washed three times with water. The filtrate was extracted with ethyl acetate (2 × 10mL) and the aqueous layer was left. 1M) adjusted to pH 1-2, extracted with ethyl acetate (3 × 20 mL), dried over anhydrous sodium sulfate, and removed the solvent to obtain m-dibenzoic acid with a yield of 65%
References:
CN110724047,2020,A Location in patent:Paragraph 0141-0143; 0159-0161; 0177
108-67-8
379 suppliers
$5.00/5G

554-95-0
447 suppliers
$6.00/25g
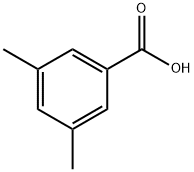
499-06-9
395 suppliers
$6.00/10g

554-95-0
447 suppliers
$6.00/25g
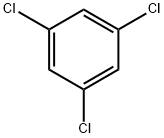
108-70-3
255 suppliers
$21.00/100g
201230-82-2
1 suppliers
inquiry
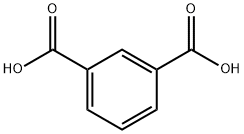
121-91-5
602 suppliers
$6.00/10g

554-95-0
447 suppliers
$6.00/25g