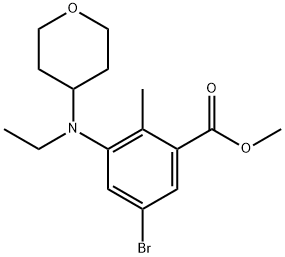
Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester synthesis
- Product Name:Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester
- CAS Number:1403257-79-3
- Molecular formula:C16H22BrNO3
- Molecular Weight:356.25
![Benzoic acid, 5-broMo-2-Methyl-3-[(tetrahydro-2H-pyran-4-yl)aMino]-, Methyl ester](/CAS/20150408/GIF/1403257-49-7.gif)
1403257-49-7
38 suppliers
$183.33/0.1g
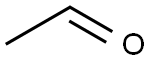
75-07-0
422 suppliers
$14.00/5mL
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
36 suppliers
inquiry
Yield: 100%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 20; for 24 h;
Steps:
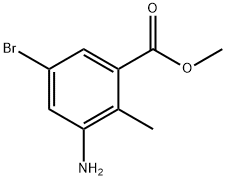
Methyl 5-bromo-3-[ethyl(oxan-4-yl)amino]-2-methylbenzoate
To a stirred solution of methyl 5-bromo-2-methyl-3-[(oxan-4-yl)amino]benzoate (39.1 g, 119 mmol) in CH2Cl2 (400 mL) and AcOH (40 mL) was added acetaldehyde (24.7 g, 476 mmol) and sodium triacetoxyborohydride (79.6 g, 357 mmol). The reaction mixture was stirred at RT for 24 hours. Then saturated NaHCO3 aq.was added and the mixture was separated. The aqueous layer was extracted with CH2Cl2 and the combined organic layer was concentrated in vacuo. The residue was purified by silica gel column chromatography (SiO2Heptane/EtOAc=3/1) to give the titled compound as a viscous oil (44.1 g, quantitative yield). 1H-NMR (400 MHz, DMSO-d6) δ ppm; 7.62 (s, 1H), 7.52 (s, 1H), 3.80 (m, 5H), 3.31 (m, 2H), 2.97-3.05 (m, 2H), 2.87-2.96 (m, 1H), 2.38 (s, 3H), 1.52-1.61 (m, 2H), 1.37-1.50 (m, 2H), 0.87 (t, J=6.8 Hz, 3H).
References:
Kuntz, Kevin Wayne;Campbell, John Emmerson;Seki, Masashi US2014/107122, 2014, A1 Location in patent:Paragraph 0191; 0192
![Benzoic acid, 5-broMo-2-Methyl-3-[(tetrahydro-2H-pyran-4-yl)aMino]-, Methyl ester](/CAS/20150408/GIF/1403257-49-7.gif)
1403257-49-7
38 suppliers
$183.33/0.1g
75-03-6
363 suppliers
$11.00/5g
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
36 suppliers
inquiry
1000342-11-9
179 suppliers
$6.00/1g
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
36 suppliers
inquiry

29943-42-8
425 suppliers
$5.00/1g
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
36 suppliers
inquiry
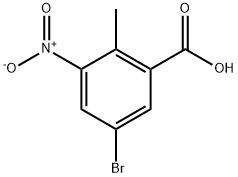
107650-20-4
157 suppliers
$9.00/250mg
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
36 suppliers
inquiry