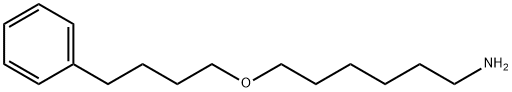
Salmeterol Impurity 9 synthesis
- Product Name:Salmeterol Impurity 9
- CAS Number:97664-58-9
- Molecular formula:C16H27NO
- Molecular Weight:249.39
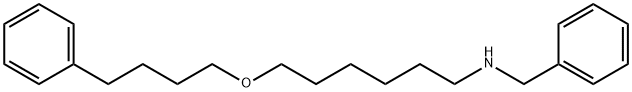
97664-55-6
125 suppliers
$375.00/25g

97664-58-9
17 suppliers
inquiry
Yield:97664-58-9 97.9%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in ethanol at 20; under 760.051 Torr;
Steps:
4 EXAMPLE 4. 6-(4-Phenylbutoxy)hexylamine
A solution of 4.02 g (11.86 mmol) N-benzyl-6-(4-phenylbutoxy)hexylamine in 40 ml EtOH is hydrogenated at room temperature and atmospheric pressure with 0.4 g 5% Pd/C. When 300 ml H2 have been absorbed, the mixture is filtered on decalite and the filtrate is concentrated to yield 2.89 g (97.9%) 6-(4-phenylbutoxy)hexylamine as an oil. RMN 1H (CDCl3), δ (ppm): 1.2-1.8 (m, 12H), 2.65 (c, 4H, -CH2-C6H5+-CH2NH2), 3.4(c, 4H, -CH2-O-CH2-)7.1-7.4(m, 5H, -C6H5).
References:
US6388134,2002,B1 Location in patent:Page column 9
![Carbamic acid, N-[6-(4-phenylbutoxy)hexyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/928118-30-3.gif)
928118-30-3
0 suppliers
inquiry

97664-58-9
17 suppliers
inquiry
![1-[4-[(6-Bromohexyl)oxy]butyl]benzene](/CAS/GIF/94749-73-2.gif)
94749-73-2
43 suppliers
inquiry

97664-58-9
17 suppliers
inquiry
![Benzene, [4-[(6-azidohexyl)oxy]butyl]-](/CAS/20211123/GIF/168412-89-3.gif)
168412-89-3
0 suppliers
inquiry

97664-58-9
17 suppliers
inquiry

629-03-8
492 suppliers
$6.00/25g

97664-58-9
17 suppliers
inquiry