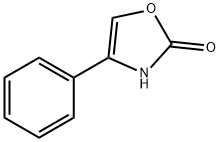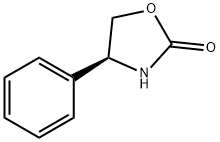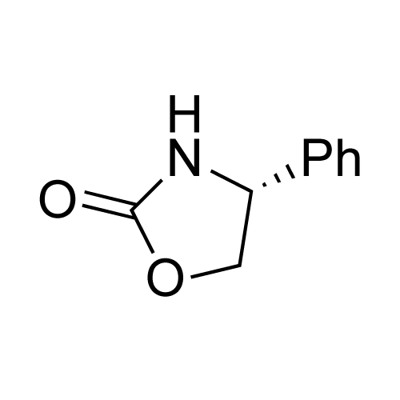
(R)-(-)-4-Phenyl-2-oxazolidinone synthesis
- Product Name:(R)-(-)-4-Phenyl-2-oxazolidinone
- CAS Number:90319-52-1
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
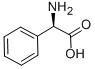
875-74-1
423 suppliers
$6.00/10g
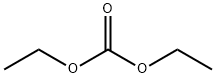
105-58-8
458 suppliers
$14.00/25g

90319-52-1
374 suppliers
$7.00/5g
Yield:90319-52-1 85%
Reaction Conditions:
with potassium carbonate at 130 - 140;
Steps:
4.2.1 Synthesis of (R,S) 4-phenyloxazolidin-2-one 13
General procedure: A dry 250ml three-necked round bottom flask equipped with a thermometer and a 10cm vigreux column with a distillation head, was charged with 4.3g of the (R,S)-phenylglycinol (31.4mmol) obtained and added of 9.3g of diethyl carbonate (79.0mmol) and 0.43g of K2CO3 (3.10mmol). The mixture was heated carefully to 130-140°C and the ethanol was distils as it formed. The oily residue was cooled and added by 50ml of CH2Cl2 to facilitate the filtration of the remaining potassium carbonate. The organic phase was then washed with a satured solution of NaHCO3, separated and dried over anhydrous Na2SO4, filtrate and evaporated in vacuo. The residue was crystallized from AcOEt/Etp (1:3) to afford 4.3g (26.4mmol, 85%) of 13 as a white solid.
References:
Zampieri, Daniele;Vio, Luciano;Fermeglia, Maurizio;Pricl, Sabrina;Wünsch, Bernhard;Schepmann, Dirk;Romano, Maurizio;Mamolo, Maria Grazia;Laurini, Erik [European Journal of Medicinal Chemistry,2016,vol. 121,p. 712 - 726]

124-38-9
129 suppliers
$175.00/23402
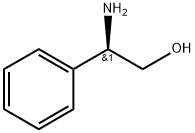
56613-80-0
526 suppliers
$5.00/1g

90319-52-1
374 suppliers
$7.00/5g