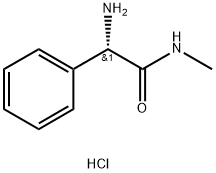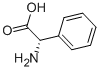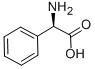
D-2-Phenylglycine synthesis
- Product Name:D-2-Phenylglycine
- CAS Number:875-74-1
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
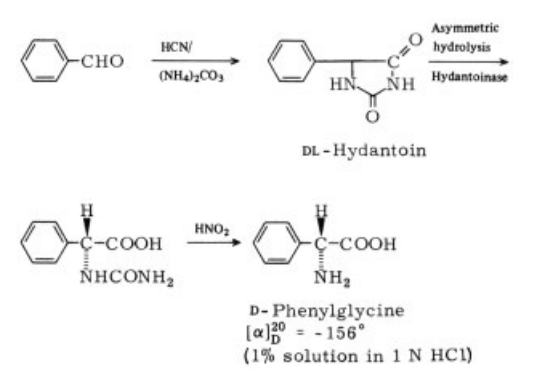
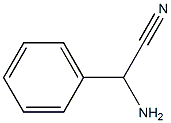
41804-94-8
1 suppliers
inquiry

875-74-1
420 suppliers
$6.00/10g
Yield:95 % ee
Reaction Conditions:
with α-aminonitrilase NIT158 from Sphingomonas wittichii in aq. phosphate buffer at 30; pH=7.3;Enzymatic reaction;stereospecific reaction;
Steps:
2.6.2. Activity of NIT28 on 2-amino-2-phenylacetonitrile (5)
General procedure: 10 mM of 2-amino-2-phenylacetonitrile (5) were incubated at 30°C in 100 mM potassium phosphate buffer pH 7.3 with 0.008 mg of purified NIT28 in a final volume of 1.0 mL. An aliquot (100 μl) of the reaction mixture was quenched after a specified period of time by addition of 1 μl of TFA, centrifugated (13,000 rpm, 10 min) to pellet the precipitated protein and 5 μl of the supernatant were directly subjected to HPLC analysis (conditions 2, tR (PheGly) = 3.78 min, tR (PheGlyCN) = 7.55 min). Conversion rates were determined by comparing enzymatic product concentration to standard curves of synthesized racemic product. For determination of the enantiomeric excess, 50 μl of the supernatant were mixed with 20 μl of 1 M K2CO3 solution and 100 μl of a solution of (S)-2-((5-fluoro-2,4-dinitrophenyl)propanamide) (FDAA) (6.4 mM in acetone). The resulting yellow solution was heated at 40°C for 1 h 30 min under stirring (1000 rpm) then quenched with HCl 1 M (20 μl), diluted with 100 μl of MeCN and filtered off before HPLC analysis (injection 10 μl; conditions 3, tR [(S)-PheGly] = 9.30 min, tR [(R)-PheGly] = 12.51 min).
References:
Bordier, Franck;Stam, Mark;Darii, Ekaterina;Tricot, Sabine;Fossey, Aurelie;Rohault, Johanna;Debard, Adrien;Mariage, Aline;Pellouin, Virginie;Petit, Jean-Louis;Perret, Alain;Vallenet, David;Salanoubat, Marcel;Weissenbach, Jean;Vergne-Vaxelaire, Carine;De Berardinis, Veronique;Zaparucha, Anne [Journal of Molecular Catalysis B: Enzymatic,2014,vol. 107,p. 79 - 88]
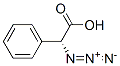
29125-25-5
4 suppliers
inquiry

875-74-1
420 suppliers
$6.00/10g
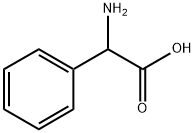
2835-06-5
286 suppliers
$6.00/25g

875-74-1
420 suppliers
$6.00/10g