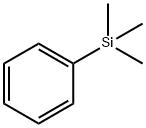
PHENYLTRIMETHYLSILANE synthesis
- Product Name:PHENYLTRIMETHYLSILANE
- CAS Number:768-32-1
- Molecular formula:C9H14Si
- Molecular Weight:150.29
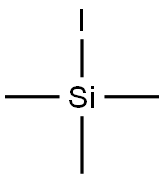
16029-98-4
408 suppliers
$28.00/5g
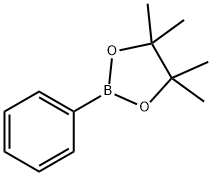
24388-23-6
212 suppliers
$6.00/5g

768-32-1
161 suppliers
$10.00/500mg
Yield:768-32-1 91%
Reaction Conditions:
Stage #1:2-phenyl-4,4,5,5-tetramethyl-1,3,2-dioxoborole with bis(tricyclohexylphosphine)nickel(II) dichloride;triethylaluminum;triethylamine in hexane;toluene;1,3,5-trimethyl-benzene at 90; for 0.5 h;
Stage #2:trimethylsilyl iodide in hexane;toluene;1,3,5-trimethyl-benzene at 90; for 24 h;Reagent/catalyst;
Steps:
1-2 Example 2
J. In a test tube with a Young valve, 14 mg (0.020 mmol) of NiCl 2 (PCy 3) 2 were added.50 μL (0.050 mmol) of 1 M triethylaluminum hexane solution and 2 mL (0.5 M) of tolueneAnd stirred at room temperature for 5 minutes to obtain a catalyst solution. Then, 224.5 mg (1.10 mmol) of phenylboronic acid pinacol ester was added to the obtained catalyst solution.280 μL (2.0 mmol) of triethylamine,And 70 μL of mesitylene (0.50 mmol: internal standard)Was added and stirred at 90 ° C. for 30 minutes to obtain a mixed solution.After allowing the mixture to cool, 138 μL (1.0 mmol) of trimethyliodosilane was added to the mixture.And reacted at 90 ° C. for 24 hours. As a result, trimethylphenylsilane was obtained at a yield of 91%.The obtained trimethylphenylsilane was measured under the same measurement conditions as in Example 1.1H-NMR measurement and 29Si-NMR measurement were performed, and the same measurement result as that of trimethylphenylsilane obtained in Example 1 was obtained.
References:
National Institute of Advanced Industrial Science and Technology;Wakebe, Teruo;Nakajima, Yumiko;Sato, Kazuhiko JP2020/7227, 2020, A Location in patent:Paragraph 0052-0054

108-86-1
519 suppliers
$10.00/5g
1450-14-2
295 suppliers
$29.12/10ML

768-32-1
161 suppliers
$10.00/500mg

2857-97-8
444 suppliers
$7.00/25g

24388-23-6
212 suppliers
$6.00/5g

768-32-1
161 suppliers
$10.00/500mg
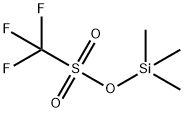
27607-77-8
507 suppliers
$10.00/5g

24388-23-6
212 suppliers
$6.00/5g

768-32-1
161 suppliers
$10.00/500mg
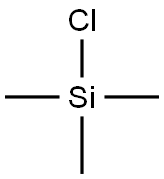
75-77-4
590 suppliers
$5.04/10
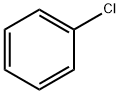
108-90-7
671 suppliers
$10.00/25G

768-32-1
161 suppliers
$10.00/500mg