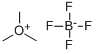
Trimethyloxonium Tetrafluoroborate synthesis
- Product Name:Trimethyloxonium Tetrafluoroborate
- CAS Number:420-37-1
- Molecular formula:C3H9BF4O
- Molecular Weight:147.91
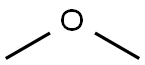
115-10-6
96 suppliers
$75.00/25g
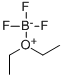
109-63-7
393 suppliers
$10.60/1gm:
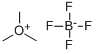
420-37-1
236 suppliers
$17.00/5g
Yield:420-37-1 98%
Reaction Conditions:
with epichlorohydrin in dichloromethane;Cooling with acetone-dry ice;Inert atmosphere;
Steps:
Preparation of trimethyloxonium tetrafluoroborate (adapted from TJ. Curphey, Org. Synth., Coll. Vol. 6, p.1019 (1988) with slight modification as follows): [00143] An oven-dried 500 mL 3 -neck round-bottomed flask (marked with a line marked at about 190 mL), equipped with a N2 inlet, 60 mL pressure- equalized dropping funnel, magnetic stirrer bar and rubber septum was placed under a blanket of dry nitrogen gas. DCM (80 mL) was added, followed by boron trifluoride diethyl etherate (33.3 mL, 270 mmol) and the mixture was cooled in a dry ice-acetone bath. Dimethyl ether was condensed into the DCM solution via a needle (through the rubber septum) that remained just below the surface until the total volume of the liquid reached the 190 mL mark. The mixture was stirred vigorously while epichlorohydrin (24.1 mL, 307 mmol) was added drop wise over approximately 15 minutes (the mixture became very thick and required occasion manual swirling to ensure good stirring). The bath was removed and the mixture was stirred vigorously overnight. The resulting solid was collected by filtration through an oven dried, medium frit glass Buchner funnel under a stream of N2 and the flask and filter were rinsed with DCM (2x100 mL). The trimethyloxonium tetrafluoroborate product was isolated as a free-flowing white solid (29.4 g, 98%) after drying under nitrogen and was stored under nitrogen in an oven-dried glass bottle in a freezer.
References:
WO2010/68520,2010,A2 Location in patent:Page/Page column 28-29

115-10-6
96 suppliers
$75.00/25g
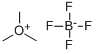
420-37-1
236 suppliers
$17.00/5g
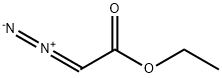
623-73-4
149 suppliers
$27.30/25ML

115-10-6
96 suppliers
$75.00/25g
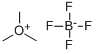
420-37-1
236 suppliers
$17.00/5g

115-10-6
96 suppliers
$75.00/25g
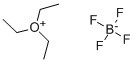
368-39-8
263 suppliers
$36.53/25ml
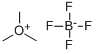
420-37-1
236 suppliers
$17.00/5g
