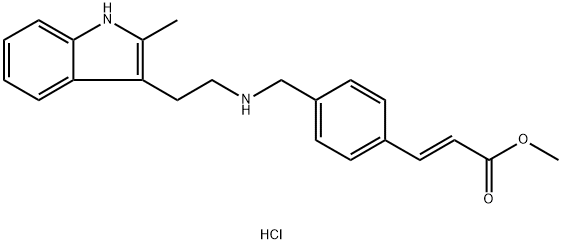
Panobinostat Carboxylic Acid Methyl Ester Hydrochloride synthesis
- Product Name:Panobinostat Carboxylic Acid Methyl Ester Hydrochloride
- CAS Number:441741-66-8
- Molecular formula:C22H25ClN2O2
- Molecular Weight:384.9

2731-06-8
139 suppliers
$50.00/1g
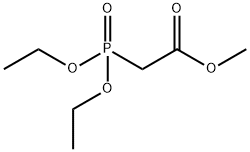
1067-74-9
216 suppliers
$5.00/5g
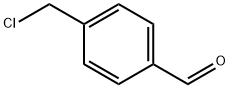
73291-09-5
113 suppliers
$65.00/100mg

441741-66-8
21 suppliers
$165.00/5mg
Yield:441741-66-8 55%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene;lithium chloride in N,N-dimethyl-formamide at 0 - 25; for 6 h;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;
Steps:
Synthesis of (E)-methyl 3-[4- ({[2- (2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylate hydrochloride (4)
A mixture of 2-(2-methyl-1H-indol-3-yl)ethanamine (1, 1.5 g, 9 mmol), DMF (10 mL), DBU (3.8 g, 25 mmol), LiCl (0.04g, 1 mmol) and methyl diethylphosphonoacetate (6, 5.2 g, 25 mmol) was stirred at 0 °C under a nitrogen atmosphere for 10 min, and then this mixture was slowly treated with a solution of 4-(chloromethyl)benzaldehyde (5, 1.5 g, 10 mmol) in DMF (5 mL). The reaction was then warmed to room temperature and stirred at this temperature for 6 h to give a red solution. Water (50 mL) was added to quench the reaction and it was extracted with ethyl acetate (2 × 50 mL). The combined organic layer was washed with brine and dried over anhydrous Na2SO4and filtered. Then saturated HCl/1,4-dioxane solution was added to this filtrate until pH = 2-3, and concentrated to dryness. The residue was stirred in hot ethyl acetate/EtOH for 10 min, and the resulting precipitate was filtered and dried to give the pure compound 4as: Brown solid; yield 1.8 g (55%); m.p. 239-243 °C (lit. 12245-247 °C); 1H NMR (600 MHz, DMSO-d6): δ: 2.34 (s, 3H), 2.92 (m, 2H), 3.17 (m, 2H), 3.74 (s, 3H), 4.21 (t, J= 5.8 Hz, 2H), 6.72 (d, J= 16.1 Hz, 1H), 7.01 (m, 2H), 7.25 (d, J= 8.0 Hz, 1H), 7.46 (d, J= 7.8 Hz, 1H), 7.65 (d, J= 8.2 Hz, 2H), 7.69 (d, J= 16.1 Hz, 1H), 7.80 (d, J= 8.2 Hz, 2H), 9.59 (brs, 2H), 10.90 (s, 1H); 13C NMR (150 MHz, DMSO-d6): δ11.6, 21.0, 47.4, 49.8, 52.0, 66.8, 105.6, 111.0, 117.6, 118.7, 119.0, 120.6, 128.2, 128.9, 130.9, 133.1, 134.9, 135.6, 144.2, 167.0; MS (ESI) m/z[M + H]+: 349.0; HRMS m/z calcd for C22H24O2N2[M + H]+: 349.1911; found: 349.1914.
References:
Chen, Shanwen;Zhang, Peiming;Chen, Huali;Zhang, Pu;Yu, Yu;Gan, Zongjie [Journal of Chemical Research,2018,vol. 42,# 9,p. 471 - 473]
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
16 suppliers
inquiry

441741-66-8
21 suppliers
$165.00/5mg