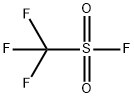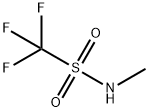
N-MethyltrifluoroMethanesulfonaMide synthesis
- Product Name:N-MethyltrifluoroMethanesulfonaMide
- CAS Number:34310-29-7
- Molecular formula:C2H4F3NO2S
- Molecular Weight:163.12
358-23-6
620 suppliers
$10.00/1g
593-51-1
562 suppliers
$21.00/100g

34310-29-7
70 suppliers
$220.00/500mg
Yield:34310-29-7 86%
Reaction Conditions:
with triethylamine in dichloromethane at -78 - 20;Inert atmosphere;
Steps:
Procedure B (from Amine Hydrochlorides) An oven dried round-bottom flask equipped with a stir bar was charged with amine hydrochloride (5.0 mmol, 1.0 equiv), CH2Cl2 (10 ml, 0.5M) and Et3N (1.53 mL, 11 mmol, 2.2 equiv). The flask was cooled to -78°C., and Trifluoromethanesulfonic anhydride (840 μL, 1.41 g, 5.0 mmol, 1.0 equiv) was added dropwise. The reaction was stirred vigorously at -78°C. for 30 min and allowed to gradually warm up to r.t. The reaction was then quenched with 20 mL H2O. The reaction mixture was partitioned between H2O and CH2Cl2 and layers were separated. The aqueous layer was extracted with CH2Cl2 (20 ml3). The organic layers were combined, dried over NaSO4, filtered, and concentrated under reduced pressure. The reaction mixture was applied directly to a flash silica column for purification (Acetone/Hexanes). NOTE: Most N-trifyl protected amines are bench stable for at least one year. (Some of them exhibited color change and suboptimal reactivity (10-15% drop in yield) after storing on bench for over 12 months, repurification by flash column usually restored reactivity in these cases.
References:
US10266503,2019,B1 Location in patent:Page/Page column 119
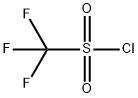
421-83-0
249 suppliers
$22.39/1G

593-51-1
562 suppliers
$21.00/100g

34310-29-7
70 suppliers
$220.00/500mg

358-23-6
620 suppliers
$10.00/1g
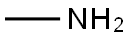
74-89-5
0 suppliers
$13.44/25ML

34310-29-7
70 suppliers
$220.00/500mg

421-83-0
249 suppliers
$22.39/1G
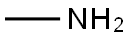
74-89-5
0 suppliers
$13.44/25ML

34310-29-7
70 suppliers
$220.00/500mg