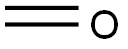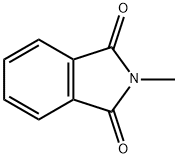
N-Methylphthalimide synthesis
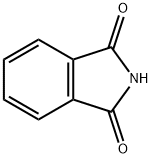
- Product Name:N-Methylphthalimide
- CAS Number:550-44-7
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
Yield:550-44-7 100%
Reaction Conditions:
with sodium methylate;sodium hydride in dimethyl sulfoxide at 20; for 10 h;
Steps:
11 Preparation of N-methylphthalimide
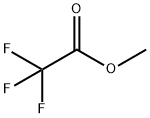
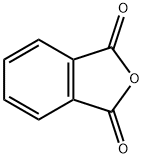
Phthalimide (100 mg, 0.68 mmol), DMSO (10 mL), CH3ONa (112 mg, 2 mmol, 3 eq.) was added to the reaction flask in turn, stirred at room temperature for 5 minutes, and then uniformly dispersed after NaH. Methyl trifluoroacetate (0.28 mL, 2.7 mmol, 4 eq.) was slowly injected into the reaction flask. After reacting for 10 hours, the TLC was plated, and the starting point disappeared, that is, the reaction was completed, and the stirring was stopped. Extract twice with ethyl acetate and distilled water, then wash with saturated NaCl solution. The organic phases were combined, and the organic phase was dried over anhydrous magnesium sulfate. The magnesium sulfate was removed by filtration, and the solvent was evaporated. Purification by column chromatography gave white solid N-methylphthalimide (109 mg,Yield 100%).
References:
CN109369506,2019,A Location in patent:Paragraph 0011
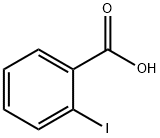
88-67-5
561 suppliers
$5.00/5g
201230-82-2
1 suppliers
inquiry
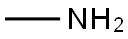
74-89-5
0 suppliers
$13.44/25ML

550-44-7
312 suppliers
$20.80/5g