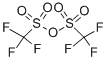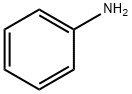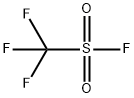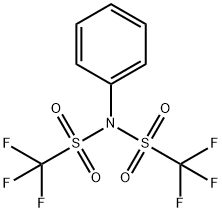
N-Phenyl-bis(trifluoromethanesulfonimide) synthesis
- Product Name:N-Phenyl-bis(trifluoromethanesulfonimide)
- CAS Number:37595-74-7
- Molecular formula:C8H5F6NO4S2
- Molecular Weight:357.25
Yield:37595-74-7 94%
Reaction Conditions:
Stage #1: trifluoromethylsulfonic anhydride;anilinewith 1,4-diaza-bicyclo[2.2.2]octane in acetonitrile at 40; under 600.06 - 1725.17 Torr; for 8 h;Sealed tube;Large scale;
Stage #2: with dmap in dichloromethane;acetonitrile; under 450.045 - 1575.16 Torr; for 15 h;Sealed tube;Large scale;
Steps:
1 Example 1
Add 9.3 kg of aniline, 40 L of acetonitrile, 13 kg of triethylenediamine to the 100L stainless steel reactor, connect the trifluoromethanesulfonyl fluoride cylinder; evacuate the reactor to -0.09Mpa, and pass in 20 kg of trifluoromethanesulfonyl fluoride Seal the reaction kettle with gas; stir the reaction for 8 hours at 40°C. Observe that the pressure of the reaction kettle gradually decreases from 0.23Mpa to 0.08Mpa and keeps no obvious change within half an hour. Open the acid fluoride recovery line to recover the excess acid fluoride. After the pressure of the reactor is reduced to 0 MPa, close the acid fluoride recovery line. Turn on the distillation system and distill off 34L of acetonitrile. Then add 40L of dichloromethane and 0.4 kg of 4-dimethylaminopyridine to the reaction kettle, and then introduce 20 kg of trifluoromethanesulfonyl fluoride again. The reaction was blocked for 15 hours, and the pressure of the reaction kettle was reduced from 0.21 MPa to 0.06 MPa. Recover excess trifluoromethanesulfonyl fluoride, distill off 38L of solvent to obtain a yellow viscous solid, then add 30 kg of ethanol, quickly stir, filter to obtain white crystals, and dry to obtain fine N-phenylbistrifluoromethanesulfonyl The amine was 33.6 kg, the detected purity was 99.2%, and the yield was 94.0%.
References:
CN111269152,2020,A Location in patent:Paragraph 0047-0050