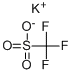
Potassium trifluoromethanesulfonate synthesis
- Product Name:Potassium trifluoromethanesulfonate
- CAS Number:2926-27-4
- Molecular formula:CF3KO3S
- Molecular Weight:188.17
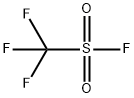
335-05-7
44 suppliers
inquiry

2926-27-4
278 suppliers
$5.00/1g
Yield:2926-27-4 1201 g
Reaction Conditions:
with potassium hydroxide;calcium hydroxide in water under 1500.15 - 7500.75 Torr; for 7 h;Reagent/catalyst;
Steps:
1; 2 Example 2
793 g of an aqueous solution of potassium hydroxide of 49.4%, 434 g of calcium hydroxide and 774 g of high purity water were successively added to a 304 stainless steel reaction vessel, the temperature was raised to 70 ° C, and the mixture was stirred for 2 hours. Then, the trifluoromethane sulfonyl fluoride gas , The reaction pressure is controlled between 0.2MPa and 1.0MPa; when the ventilation quality is 980g trifluoromethanesulfonyl fluoride, the aeration time is 3 hours, then the reaction is kept for 2 hours, then the temperature is lowered to 25 DEG C; And the mixture was filtered to obtain an aqueous potassium trifluoromethanesulfonate solution having a concentration of 48.74% of potassium trifluoromethanesulfonate, pH = 14, F = 4 ppm, ICP detection: 0.4 ppm of Fe, and 0.09 ppm of Ni. The fluoride ion concentration <10ppm, Fe <1ppm, Ni <1ppm, indicating that the reaction of 304 stainless steel without corrosion, the method greatly reduces the corrosion of equipment, equipment can greatly extend the service life and maintenance costs.
References:
JIANGSU GUOTAI SUPER POWER NEW MATERIALS CO., LTD.;Zou, Kai;Ren, Qidou;He, Yonggang;Qian, Xiaobing;Li, Jianzhong CN105693561, 2016, A Location in patent:Paragraph 0023; 0024; 0026; 0034
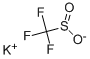
41804-89-1
29 suppliers
$110.00/1g

2926-27-4
278 suppliers
$5.00/1g
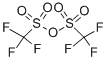
358-23-6
592 suppliers
$10.00/1g

2926-27-4
278 suppliers
$5.00/1g
2794-60-7
126 suppliers
$22.00/5g

2926-27-4
278 suppliers
$5.00/1g