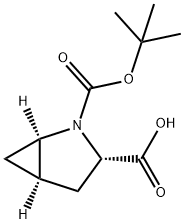
N-Boc-L-trans-4,5-Methanoproline synthesis
- Product Name:N-Boc-L-trans-4,5-Methanoproline
- CAS Number:197142-34-0
- Molecular formula:C11H17NO4
- Molecular Weight:227.26
24424-99-5
864 suppliers
$13.50/25G
1312338-82-1
12 suppliers
inquiry

197142-34-0
101 suppliers
$28.00/100mg
Yield:197142-34-0 79%
Reaction Conditions:
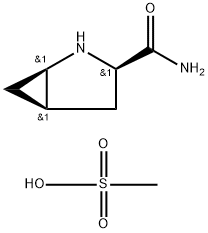
Stage #1: D-cis-4,5-methanoprolineamide methanesulfonic acid saltwith sodium ethanolate in ethanol;isopropyl alcohol at 50; for 1 h;
Stage #2: with water in ethanol;isopropyl alcohol at 60; for 18 h;
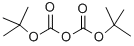
Stage #3: di-tert-butyl dicarbonate in ethanol;water;isopropyl alcohol;
Steps:
24
A I L round bottom flask equipped with a nitrogen inlet, overhead agitator, thermocouple and heating mantle was charged with 50 g (225 mmol) (lR,3R,5R)-2- Azabicyclo[3.1.0]hexane-3-carboxamide (.CH3SO3H) and 250 mL isopropanol. The resulting slurry was then charged with 252 mL of 23 wt% NaOEt in EtOH (2.68 M, 675 mmol, 3.0 equiv) and stirred at 50 °C for ca. lh. The mixture was charged with 12.2 mL (675 mmol, 3 equiv) of water and heated to 60 °C. The resulting slurry was allowed to stir at 60 °C for ca. 18h. The slurry was cooled to rt and charged with 250 mL water and 98.2 g (450 mmol, 2.0 equiv) di-/-butyldicarbonate. Ethanol and isopropanol were removed via vacuum distillation and the aqueous mixture cooled to 0 °C. The mixture was neutralized with 76 ml (456 mmol) 6M aqueous HC1 while maintaining an internal temperature < 5 °C. The product was extracted with 500 mL MTBE and the rich organic layer was washed with 100 mL water. The clear solution was concentrated down to 150 mL via vacuum distillation and the resulting slurry was charged with 600 mL heptane while maintaining an internal temperature >45 °C. The slurry was cooled to rt over ca. 30 min and allowed to stir at rt for ca. 2h. The product was filtered, washed with 250 mL 4: 1 heptane :MTBE and dried under vacuum at 70 °C to give 40.5 g (178 mmol, 79% yield, 99.8 AP at 205 nm) of (lR,3S,5R)-2-(tert-butoxycarbonyl)-2- azabicyclo[3.1.0]hexane-3-carboxylic acid: 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H), 4.02-3.80 (m, 1H), 3.45-3.15 (m, 1H), 2.40-2.19 (m, 1H), 2.19-2.0 (m, 1H), 1.70- 1.50 (m, 1H), 1.50-1.20 (m, 9H), 0.83-0.60 (m, 1H), 0.33-0.55 (m, 1H); 13C MR (100 MHz, DMSO-d6) δ 173.7, 173.2, 155.0, 154.3, 79.4, 60.5, 60.2, 37.6, 32.6, 31.8, 28.4, 28.2, 15.6, 15.2, 14.4; HRMS calcd for C11H18N04 (M + H; ESI+): 228.1236. Found: 228.1234.
References:
WO2011/59850,2011,A1 Location in patent:Page/Page column 179-180
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry

197142-34-0
101 suppliers
$28.00/100mg
![(3S)-3-((tert-butyldiphenylsilyloxy)methyl)-2-(tert-butoxycarbonyl)-2-azabicyclo[3.1.0]hexane](/CAS/20180703/GIF/1208008-34-7.gif)
1208008-34-7
4 suppliers
inquiry

197142-34-0
101 suppliers
$28.00/100mg
![(3S)-3-((tert-butyldiphenylsilyloxy)methyl)-2-(tert-butoxycarbonyl)-2-azabicyclo[3.1.0]hexane](/CAS/20180703/GIF/1208008-34-7.gif)
1208008-34-7
4 suppliers
inquiry
![2-Azabicyclo[3.1.0]hexane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1R,5R)-](/CAS/20210305/GIF/1310363-30-4.gif)
1310363-30-4
1 suppliers
inquiry

197142-34-0
101 suppliers
$28.00/100mg
![(1S,3S,5S)-2-(TERT-BUTOXYCARBONYL)-2-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLIC ACID](/StructureFile/ChemBookStructure20/GIF/CB2825254.gif)
197142-36-2
74 suppliers
$98.00/100mg
![tert-butyl (3S)-3-[[(tert-butyldiphenylsilyl)oxy]methyl]-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180703/GIF/197142-32-8.gif)
197142-32-8
2 suppliers
inquiry

197142-34-0
101 suppliers
$28.00/100mg