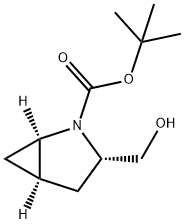
tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate synthesis
- Product Name:tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate
- CAS Number:197142-33-9
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
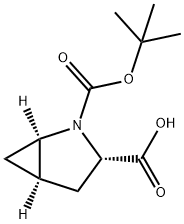
197142-34-0
95 suppliers
$28.00/100mg
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry
Yield:197142-33-9 91%
Reaction Conditions:
Stage #1: (1R,3S,5R)-2-(tert-butoxycarbonyl)-2-azabicyclo[3.1.0]hexane-3-carboxylic acidwith borane-THF in tetrahydrofuran at 0; for 3.5 h;Reflux;
Stage #2: with methanol in tetrahydrofuran at 0;
Steps:
61
To a solution of (lR,3S,5R)-2-(tert-butoxycarbonyl)-2- azabicyclo[3.1.0]hexane-3-carboxylic acid (9.85 g, 43.3 mmol) in THF (200 mL) at 0 °C was added dropwise borane-methyl sulfide complex (282 mL, 563 mmol) over 30 min. The ice bath was removed, the mixture was stirred for lh and then heated at reflux for 2h. The mixture was cooled to 0 °C, slowly quenched with methanol (-200 mL) and concentrated under vacuum. The residue was dissolved in DCM and washed with water (emulsion), IN HC1, sat NaHCC aq, and brine. The organic layer was dried over a2S04, filtered and concentrated to yield (lR,3S,5R)-/er/-butyl 3-(hydroxymethyl)-2- azabicyclo[3.1.0]hexane-2-carboxylate (8.43 g, 39.5 mmol, 91 % yield) as colorless oil. LC-MS retention time 1.398 min; m/z 236.20 [M+Na]+. LC data was recorded on a Shimadzu LC-10AS liquid chromatograph equipped with a Waters Sunfire 5u CI 8 4.6x30mm column using a SPD-10AV UV-Vis detector at a detector wave length of 220nM. The elution conditions employed a flow rate of 4 mL/min, a gradient of 100% Solvent A / 0% Solvent B to 0% Solvent A / 100% Solvent B, a gradient time of 2 min, a hold time of 1 min and an analysis time of 4 min where Solvent A was 10% methanol / 90% water / 0.1% TFA and Solvent B was 90% methanol/ 10% water / 0.1% TFA. MS data was determined using a MICROMASS Platform for LC in electrospray mode. 1H NMR (500 MHz, MeOD) δ ppm 3.72 - 3.79 (m, 1H), 3.52 - 3.64 (m, 3H), 3.15 - 3.24 (m, 1H), 2.00 - 2.08 (m, 1H), 1.62 - 1.72 (m, 1H), 1.54 - 1.62 (m, 1H), 1.45 - 1.51 (m, 9H), 0.84 (br s, 1H), 0.36 (td, J=5.0, 2.4 Hz, 1H).
References:
WO2011/59850,2011,A1 Location in patent:Page/Page column 215
![tert-butyl (3S)-3-[[(tert-butyldiphenylsilyl)oxy]methyl]-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180703/GIF/197142-32-8.gif)
197142-32-8
2 suppliers
inquiry
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry
![(2S)-2-[[[(1,1-DIMETHYLETHYL)DIPHENYLSILYL]OXY]METHYL]-5-OXO-1-PYRROLIDINE](/CAS/GIF/132974-83-5.gif)
132974-83-5
15 suppliers
inquiry
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry
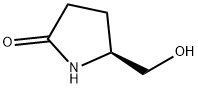
17342-08-4
332 suppliers
$5.00/1g
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry

24424-99-5
839 suppliers
$13.50/25G
![tert-butyl (3S)-3-(hydroxymethyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/20180702/GIF/197142-33-9.gif)
197142-33-9
11 suppliers
inquiry