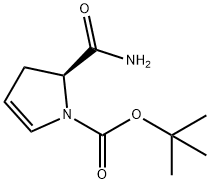
1H-Pyrrole-1-carboxylic acid, 2-(aMinocarbonyl)-2,3-dihydro-, 1,1-diMethylethyl ester, (2S)- synthesis
- Product Name:1H-Pyrrole-1-carboxylic acid, 2-(aMinocarbonyl)-2,3-dihydro-, 1,1-diMethylethyl ester, (2S)-
- CAS Number:709031-38-9
- Molecular formula:C10H16N2O3
- Molecular Weight:212.25
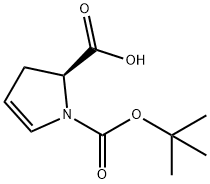
90104-21-5
19 suppliers
inquiry

709031-38-9
54 suppliers
inquiry
Yield:709031-38-9 359.7 g
Reaction Conditions:
with 4-methyl-morpholine;ammonia;methanesulfonyl chloride at -15 - -8;
Steps:
(S)-tert-Butyl 2-carbamoyl-2,3-dihydro-1H-pyrrole-1-carboxylate (4)
Compund 7 (415.0 g, 1.9 mol)was slurried in 2.1 L MeOH/water (2:1), lithium hydroxide monohydrate (119.6 g, 2.85 mol) was addedin portions while maintaining the batch temperature in the range of -5-0°C. After complete addition ofthe lithium hydroxide monohydrate, the reaction mixture was heated to 25-30°C and stirring for 1 h,MeOH was removed by vacuum distillation and the aqueous layer was back-extracted with methyltert-butyl ether. Add 1 L methyl tert-butyl ether to the aqueous layer and then add 20% phosphoric acid solution dropwise to adjust pH to 2-3. Layers were separated, the aqueous layer was extracted with 400mL methyl tert-butyl ether. The combined organic phase was dried and filtered, methyl tert-butyl ethersolution of intermediate was obtained, and the solution can be used in the next step directly. Transferred the methyl tert-butyl ether solution to a reaction vessel, cooled to -15 °C. Added N-methylmorpholine(252.5 g, 2.5 mol) and MsCl (228 g, 2 mol) dropwise respectively, while maintaining the batch temperature no more than -8 °C. The reaction mixture was stirred for 0.5 h under this temperaure. Ammonia gas was bubbled into and stirred for 1.5 h. Added saturated aqueous NaCl solution (1 L) to thesolution and stirred for 1 h. Then add 20% phosphoric acid solution dropwise to adjust pH to 5-6. Theorganic layer washed with saturated aqueous NaHCO3 (500 mL×2)dried over anhydrous magnesiumsulfate. Distilled off the solvent completely from the organic layer under reduced pressure to get the titlecompound 4 as pale yellow viscous, recrystallized from i-PrOH to form a off white solid (359.7 g, HPLC:98.4%, yield: 89.2 % for 2 steps). 1H NMR (500 MHz, CDCl3) δ 7.31 (m, 1H), 6.88 (br, 1H), 6.48-6.45(m, 1H), 4.95 (m, 1H), 4.34 (dd, J = 12.5, 6.0 Hz, 1H), 2.86-3.00 (m, 1H), 2.44-2.51 (m, 1H), 1.41 (s, 9H).ESI-MS m/z: 213.2 (M+1)+.
References:
Ding, Ding;Pan, Xianhua;Yu, Wansheng;Li, Xiaojun;Chen, Suke;Liu, Feng [Heterocycles,2015,vol. 91,# 4,p. 719 - 726]
709031-37-8
0 suppliers
inquiry

709031-38-9
54 suppliers
inquiry
178172-26-4
109 suppliers
$144.00/250mg

709031-38-9
54 suppliers
inquiry
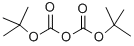
24424-99-5
839 suppliers
$13.50/25G

709031-38-9
54 suppliers
inquiry
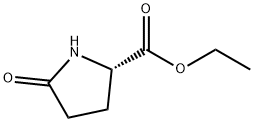
7149-65-7
238 suppliers
$15.00/25G

709031-38-9
54 suppliers
inquiry