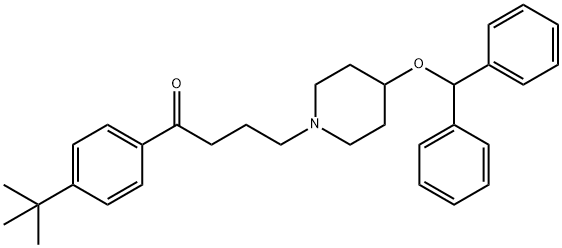
Ebastine synthesis
- Product Name:Ebastine
- CAS Number:90729-43-4
- Molecular formula:C32H39NO2
- Molecular Weight:469.66
5382-16-1
0 suppliers
$9.00/5 g
119-61-9
806 suppliers
$5.00/10g
43076-61-5
239 suppliers
$71.00/25g

90729-43-4
386 suppliers
$35.00/100mg
Yield:90729-43-4 31 g
Reaction Conditions:
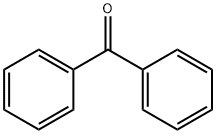
Stage #1:benzophenone with aluminum (III) chloride;trifluorormethanesulfonic acid in toluene at 50; for 0.333333 h;
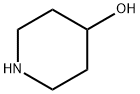
Stage #2:4-HYDROXYPIPERIDINE with toluene-4-sulfonic acid in water;toluene for 5 h;Reflux;
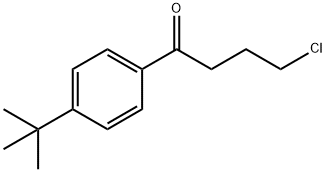
Stage #3:4'-tert-butyl-4-chlorobutyrophenone with sodium hydrogencarbonate in water;toluene for 12 h;Reflux;Solvent;Reagent/catalyst;
Steps:
1-3 Example 1
The preparation method of ebastine provided in this embodiment specifically includes the following steps: (1) Add benzophenone (30 g, 0.165 mol) to 300 mL of toluene solution, stir and dissolve, aluminum trichloride (2.2 g, 0.017 mol) was added in sequence,trifluoromethanesulfonic acid (2.5 g, 0.018 mol),the mixture was heated to 50 ° C for 20 min, cooled to room temperature, p-toluenesulfonic acid (31.2 g, 0.18 mol) was added, 4-hydroxypiperidine (17.2 g, 0.17 mol) was added, and the mixture was heated to reflux, and refluxed with water for 5 h. After the reaction was completed, the reaction solution was cooled to room temperature, and 230 mL of 1N NaOH solution was slowly added thereto under ice cooling, and the mixture was stirred for 30 minutes, left to stand for 30 minutes, and the aqueous layer was separated. The organic layer was further added with sodium hydrogencarbonate (35 g, 0.41 mol). ,4-chloro-1-(4-tert-butylphenyl)-1-butanone (37.5 g, 0.157 mol), warmed to reflux, and refluxed with water for 12 h. After the reaction was completed, the temperature was lowered to room temperature and washed three times. , 200mL each time, wash to neutral.(2) The obtained organic solution was evaporated to the remaining 10% volume, 100 mL of 95% ethanol was added, the temperature was dissolved, the temperature was lowered to -10 ° C, and the crystal was frozen for 12 hours. The next day, the crystal was filtered off and dried to obtain ebastine (31g) 0.067mol), mp 84.2-86.3°C, total yield 40%
References:
Jiangsu Lianhuan Pharmaceutical Co., Ltd.;Wu Weibi CN109593058, 2019, A Location in patent:Paragraph 0052-0064
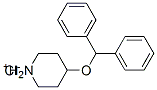
65214-86-0
72 suppliers
$65.00/100 mg

43076-61-5
239 suppliers
$71.00/25g

90729-43-4
386 suppliers
$35.00/100mg

58258-01-8
89 suppliers
$137.00/100mg

43076-61-5
239 suppliers
$71.00/25g

90729-43-4
386 suppliers
$35.00/100mg

5382-16-1
0 suppliers
$9.00/5 g

90729-43-4
386 suppliers
$35.00/100mg

43076-61-5
239 suppliers
$71.00/25g

90729-43-4
386 suppliers
$35.00/100mg