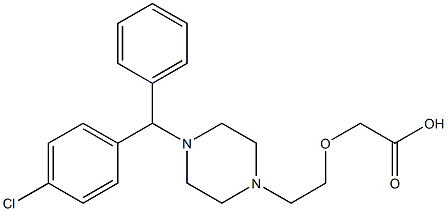
Cetirizine synthesis
- Product Name:Cetirizine
- CAS Number:83881-51-0
- Molecular formula:C21H25ClN2O3
- Molecular Weight:388.89
![Piperazine, 1-[(4-chlorophenyl)phenylmethyl]-4-[2-(2,2-diethoxyethoxy)ethyl]-](/CAS/20210305/GIF/290362-10-6.gif)
290362-10-6
0 suppliers
inquiry

83881-51-0
207 suppliers
inquiry
Yield:83881-51-0 85.3%
Reaction Conditions:
Stage #1:2-{2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy}acetaldehyde diethylacetal with hydrogenchloride in water at 60; for 0.5 h;
Stage #2: with sodium hydroxide;dihydrogen peroxide in ethanol;water; pH=6.5 - 7 at 20;
Steps:
1.B
2-{2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy}acetaldehyde diethylacetal (18.2 g of the crude product from A above, 0.0413 mol), water (20 ml) and conc. hydrochloric acid (8.3 ml, 0.1 mol) were mixed, heated for 30 min at 60 °C and then cooled to room temperature. Ethanol (20 ml) was added, and the solution was brought to pH ca. 7 with conc. sodium hydroxide solution (appr. 7 ml). To the cooled suspension (20 °C) hydrogenperoxide (4.0 ml 35 %, 0.041 mol) was added, and the resulting suspension was stirred at room temperature keeping the pH of the solution at appr. 6.5 by addition of concentrated sodium hydroxide solution. The reaction was monitored by means of HPLC (nucleosil C18, MeOH/H20 9:1, buffer: phosphate buffer pH 7, flow = 0.9 ml/min, 230 nm), and when the aldehyde (Rf = 5.39) was almost consumed, pH was adjusted to 9 with concentrated sodium hydroxide solution and the reaction was freed of peroxide with sodium dithionite (1.0 g, 0.0057 mol). The ethanol was evaporated in vacuo leaving an aqueous suspension which was diluted with water (250 ml). pH was adjusted to 9.5 with concentrated sodium hydroxide solution and the aqueous solution was extracted with butyl acetate/THF 5:1 (2 x 40 ml) at 50 °C. pH was adjusted to 1.5 with concentrated hydrochloric acid and the aqueous solution was extracted at 50 °C with butyl acetate (2 x 30 ml) and finally with cyclohexane (40 ml). The aqueous solution was cooled to room temperature, pH adjusted to 4.5 with concentrated sodium hydroxide solution, and the solution was extracted with dichloromethane (2 x 70 ml). The combined organic phases were washed with water (20 ml), dried (MgSO4) and evaporated in vacuo leaving a colourless foam (14.4 g). The foam was taken up in toluene (75 ml), heated to appr. 70 °C, and cyclohexane (25 ml) was added. Cooling overnight caused crystallisation, and the formed solid was filtered off and washed with toluene/cyclohexane 1:1 (40 ml). After drying in vacuo 13.7 g (85.3 %) of 2-{2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy}acetic acid was obtained. M. P. 135-138 °C. 1H NMR (CDCl3, 300 MHz) δ ppm: 7.1 (m, 9H); 4.20 (s, 1H); 3.95 (s, 2H); 3.70 (m, 2H); 3.00 (s, 4 H); 2.85 (m, 2H); 2.60 (s, 4H).
References:
A/S GEA Farmaceutisk Fabrik EP1157016, 2003, B1 Location in patent:Page/Page column 5
![2-[2-[4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]ethanol hydrochloride](/CAS/GIF/1244-76-4.gif)
1244-76-4
18 suppliers
inquiry

83881-51-0
207 suppliers
inquiry
![4-[(4-CHLOROPHENYL)PHENYLMETHYL]-1-PIPERAZINEETHANOL DIHYDROCHLORIDE](/CAS/GIF/109806-71-5.gif)
109806-71-5
89 suppliers
$65.00/500 mg
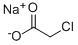
3926-62-3
298 suppliers
$16.69/250g

83881-51-0
207 suppliers
inquiry
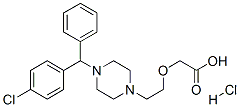
83881-52-1
671 suppliers
$5.00/100mg

83881-51-0
207 suppliers
inquiry
![4-[(4-CHLOROPHENYL)PHENYLMETHYL]-1-PIPERAZINEETHANOL DIHYDROCHLORIDE](/CAS/GIF/109806-71-5.gif)
109806-71-5
89 suppliers
$65.00/500 mg
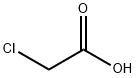
79-11-8
0 suppliers
$24.00/25g

83881-51-0
207 suppliers
inquiry