Diphenyl ether synthesis
- Product Name:Diphenyl ether
- CAS Number:101-84-8
- Molecular formula:C12H10O
- Molecular Weight:170.21
Yield:101-84-8 100%
Reaction Conditions:
with Cs2CO3;copper oxide (I);trans-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine in acetonitrile at 82; for 24 h;Conversion of starting material;activated 3 A molecular sieve (KnNa12-n[(AlO2)12(SiO2)12]);
Steps:
3.1 Example 3.1; Preparation of Diphenyl Ether
[0821] 14.4 mg of cuprous oxide (0.1 mmoles), 117 mg of Chxn-Py-Al (0.4 mmoles), 188 mg of phenol (2 mmoles), 1.303 g of caesium carbonate (4 mmoles) and 600 mg of ground and activated 3 A molecular sieve (KnNa12-n[(AlO2)12(SiO2)12]) were successively introduced into a 35 ml Schlenk tube that had been oven dried at 100° C. and provided with a magnetic stirrer (12×4.5 mm) and under a nitrogen atmosphere. [0822] The Schlenk tube was purged under vacuum then refilled with nitrogen. [0823] 336 μl of iodobenzene (3 mmoles) then 1.2 ml of acetonitrile were added using syringes. [0824] The reactor was placed in an oil bath at a temperature of 82° C. and stirred for a period of 24 hours. [0825] The degree of transformation and the selectivity for diphenyl ether were 100%. [0826] After this period, the reaction mixture was diluted with 25 ml of dichloromethane, filtered through celite, concentrated completely under reduced pressure then taken up in 50 ml of dichloromethane. [0827] This organic phase was extracted with distilled water (2×20 ml). [0828] The aqueous phase was extracted again with 20 ml of dichloromethane. [0829] The total organic phase was washed with a saturated aqueous sodium chloride solution (2×20 ml), dried over MgSO4, filtered and concentrated under reduced pressure. [0830] The oily residue obtained after treatment was complete was purified by silica gel chromatography (eluent: hexane). [0831] 344 mg of a colourless oil was obtained (corresponding to a yield of 100%), which crystallised out after a few hours in the refrigerator (colourless crystals). [0833] The characteristics were as follows: [0834] M.Pt: 26° C. (Lit: 85° C., given by Byers, C H; Williams, D F; J. Chem. Eng. Data 1987, 32, 344-348); [0835] 1H NMR/CDCl3: δ 7.37-7.47 (m, 4H, H2,4,9,11), 7.10-7.23 (m, 6H, H1,3,5,10,12); [0836] 13C NMR/CDCl3: δ 157.38 (C6 and C7), 129.88 (C2, C4, C9 and C11), 123.35 (C3 and C10), 119.02 (C1, C5, C8 and C12); [0837] GC/MS: Rt=14.43 min, M/Z=170, purity=99%; [0838] Rf: 0.33 (eluent: hexane).
References:
US2003/236413,2003,A1 Location in patent:Page 30
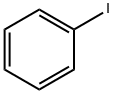
101-55-3
258 suppliers
$8.00/5g

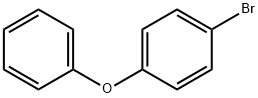
101-84-8
597 suppliers
$5.00/25g
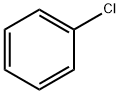
108-90-7
670 suppliers
$10.00/25G
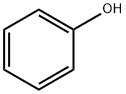
108-95-2
763 suppliers
$14.00/25g

101-84-8
597 suppliers
$5.00/25g

108-86-1
518 suppliers
$10.00/5g
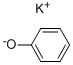
100-67-4
64 suppliers
$45.00/500mg

101-84-8
597 suppliers
$5.00/25g