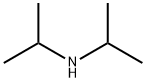
Diisopropylamine synthesis
- Product Name:Diisopropylamine
- CAS Number:108-18-9
- Molecular formula:C6H15N
- Molecular Weight:101.19

67-63-0
1610 suppliers
$17.00/25ML
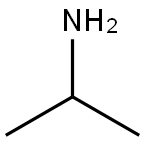
75-31-0
439 suppliers
$14.00/25mL

108-18-9
382 suppliers
$10.00/1g
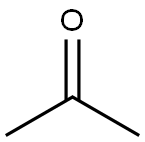
67-64-1
0 suppliers
$19.10/10ml
Yield:75-31-0 59.9%
Reaction Conditions:
with ammonia;hydrogen at 160; under 760.051 Torr; for 0.5 h;Catalytic behavior;Flow reactor;Inert atmosphere;Reagent/catalyst;
Steps:
2.4 Catalytic Activity Tests
General procedure: All catalytic experiments were performed under atmosphericpressure at continuous-flow in a fixed bedmicroreactor. Prior to testing, the catalysts were activatedat 600 °C for 3 h under a flow of purified H2 (50 cm3min-1) and kept at the desired temperature to establish astandard operating procedure, allowing time for productdistribution to stabilize. Standard conditions involved thefollowing reaction parameters: T = 160 °C; weight hourlyspace velocity (WHSV, h-1) = 4.3; and feed composition(IPA/NH3/H2/N2; molar ratio) = 1/4/6/22.8. The partialpressure of IPA was fixed at 3 kPa. For the comparison of selectivities of MIPA over variousNi/Al2O3 catalysts, space velocity was controlled in therange 2.3-19.1 at 150 °C. To maintain constant partialpressures of the reactants, nitrogen was used as a diluent.The reaction products were analyzed on-line using aChrompack-CP-9001 gas chromatograph equipped with aCP-Volamine capillary column (60 mm x 0.32 mm) and aflame ionization detector. The conversion of IPA, selectivityand WHSV were defined as follows: where FIPA and Fi are molar flow rates of IPA and i productswhich are MIPA, diisopropylamine (DIPA), acetone,respectively. MWIPA is a molecular weight of IPA. Ci is acarbon number of i products.
References:
Cho, Jun Hee;An, Sang Hee;Chang, Tae-Sun;Shin, Chae-Ho [Catalysis Letters,2016,vol. 146,# 4,p. 811 - 819]

67-63-0
1610 suppliers
$17.00/25ML

75-31-0
439 suppliers
$14.00/25mL

108-18-9
382 suppliers
$10.00/1g
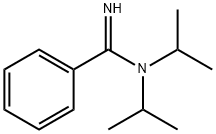
111515-28-7
0 suppliers
inquiry
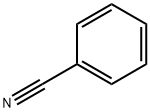
100-47-0
375 suppliers
$14.14/25gm:

108-18-9
382 suppliers
$10.00/1g
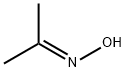
127-06-0
425 suppliers
$5.67/25g

108-18-9
382 suppliers
$10.00/1g