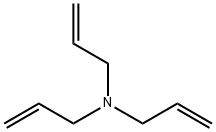
Triallylamine synthesis
- Product Name:Triallylamine
- CAS Number:102-70-5
- Molecular formula:C9H15N
- Molecular Weight:137.22
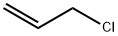
107-05-1
420 suppliers
$16.80/100ML

102-70-5
197 suppliers
$20.00/25mL

107-11-9
0 suppliers
$26.00/25ml
124-02-7
262 suppliers
$14.00/5mL
Yield:-
Reaction Conditions:
Stage #1:3-chloroprop-1-ene with formaldehyd;ammonia in methanol;water at 40 - 70; for 6 h;
Stage #2: with hydroxyammonium sulfate;sulfuric acid in methanol;water; pH=0.8 at 40; for 0.5 h;
Stage #3: with sodium hydroxide in methanol;water; pH=12Product distribution / selectivity;
Steps:
1
A stainless autoclave was charged with 3.91 parts by weight of allyl chloride (content: 98% by weight), 4.89 parts by weight of paraformaldehyde (content: 92% by weight) and 28.4 parts by weight of an aqueous 12 wt% ammonia/methanol solution, and the mixture was stirred at 40°C for 3 hours, at 50°C for 2 hours, and further at 70°C for 1 hour to react them. To the reaction mixture were added 51.3 parts by weight of an aqueous 24 wt% hydroxylamine sulfate solution and 22.7 parts by weight of 35 wt% sulfuric acid to adjust the solution to pH 0.8, followed by stirring at 40°C for 30 minutes. Then, 61.3 parts by weight of an aqueous 27 wt% sodium hydroxide solution was added to the solution to adjust the solution to pH 13. When the mixture obtained was analyzed, the yield of allylamine was 87.8%, the yield of diallylamine was 3.5%, and the yield of triallylamine was 1.2% (GC method, based on allyl chloride).
References:
Sumitomo Chemical Company, Limited EP1967509, 2008, A1 Location in patent:Page/Page column 6
591-87-7
196 suppliers
$10.00/10g

124-02-7
262 suppliers
$14.00/5mL

102-70-5
197 suppliers
$20.00/25mL
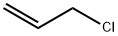
107-05-1
420 suppliers
$16.80/100ML

102-70-5
197 suppliers
$20.00/25mL

463-49-0
50 suppliers
$125.00/10 g

102-70-5
197 suppliers
$20.00/25mL

107-11-9
0 suppliers
$26.00/25ml

124-02-7
262 suppliers
$14.00/5mL
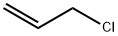
107-05-1
420 suppliers
$16.80/100ML

102-70-5
197 suppliers
$20.00/25mL