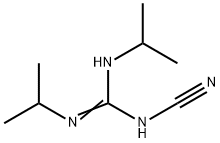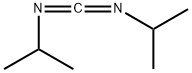
N,N'-Diisopropylcarbodiimide synthesis
- Product Name:N,N'-Diisopropylcarbodiimide
- CAS Number:693-13-0
- Molecular formula:C7H14N2
- Molecular Weight:126.2
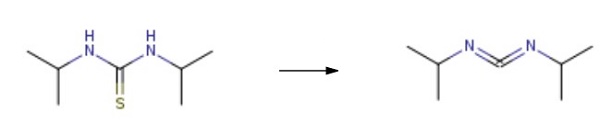
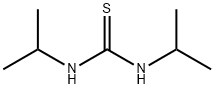
2986-17-6
220 suppliers
$7.00/25g
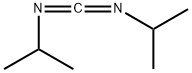
693-13-0
550 suppliers
$13.00/1ml
Yield:693-13-0 99%
Reaction Conditions:
with cyclopentadienyl iron(II) dicarbonyl dimer in tetrahydrofuran at 60; for 24 h;Inert atmosphere;Reagent/catalyst;
Steps:
3-3 Examples 3-1 to 3-9 (Synthesis of various carbodiimide compounds)
To the reaction vessel, 0.3 mmol of the thiourea compound shown in Table 3, 0.9 mmol of [CpFe (CO) 2] 2 as in Example 1-2 as an iron compound and 9 mL of tetrahydrofuran were added and the mixture was stirred at 60 ° C. under a nitrogen atmosphere And allowed to react for 24 hours. However, only in Example 3-9, each charged amount was multiplied by 100/3 and carried out.After completion of the reaction, the resulting reaction solution was analyzed with a gas chromatograph mass spectrometer (GC-MS), and it was found that the product shown in Table 3 was produced at the yield shown in Table 3.The reaction solutions obtained in Examples 3-1 to 3-9 were cooled to room temperature, water was added, and the product was extracted with ethyl acetate. Subsequently, the organic layer was washed successively with water and saturated brine, dried over anhydrous magnesium sulfate, then filtered and concentrated to obtain a crude product, and the crude product was used in Examples 3-1 to 3-8, silica gel (Developing solvent: ethyl acetate / hexane (mixing ratio was appropriately adjusted)), and purified by distillation in Example 3-9 to obtain the respective products
References:
JP5999596,2016,B2 Location in patent:Paragraph 0054-0059; 0062; 0063; 0064
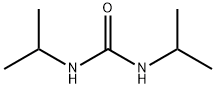
4128-37-4
79 suppliers
$50.00/5mg
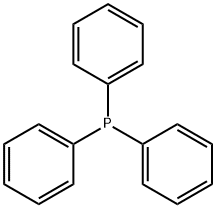
603-35-0
716 suppliers
$5.00/5G
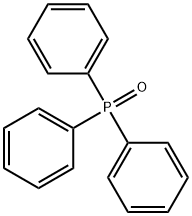
791-28-6
519 suppliers
$6.00/25g
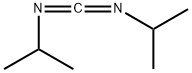
693-13-0
550 suppliers
$13.00/1ml
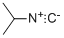
598-45-8
28 suppliers
$165.00/500mg
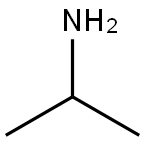
75-31-0
429 suppliers
$14.00/25mL
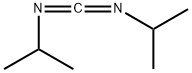
693-13-0
550 suppliers
$13.00/1ml
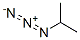
691-57-6
17 suppliers
inquiry
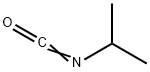
1795-48-8
177 suppliers
$24.73/250mgs:
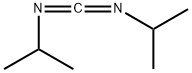
693-13-0
550 suppliers
$13.00/1ml