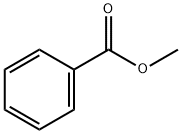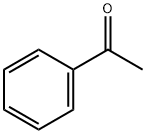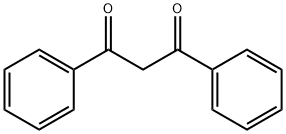
Dibenzoylmethane synthesis
- Product Name:Dibenzoylmethane
- CAS Number:120-46-7
- Molecular formula:C15H12O2
- Molecular Weight:224.25
Yield:120-46-7 99.7%
Reaction Conditions:
with sodium methylate under 225.023 Torr; for 1.25 h;Inert atmosphere;Reflux;Microwave irradiation;Claisen Condensation;Concentration;Solvent;
Steps:
5 EXAMPLE 5Synthesis of DiBenzoylMethane (DBM) using the Process According to the Invention with No Solvent
10121] The experimental apparatus consists of a classic glass, double jacketed chemical engineering reactor with volume of 1 litre with an effective mixing system. This is topped with a separating column fitted with a variable-reflux condenser. It also has a recirculating loop fitted with a gear- type pump, a 600 W microwave generator and a vacuum pump capable of reducing the pressure of the system to about 100 mbatj0122] Add 683.52 g thsed methyl benzoate and 34.00 g powdered sodium methoxide. Once the reagents have been added, render the reactor inert with a slow continuous flow of nitrogen gas maintaining a partial vacuum at about 300 mbar. Recirculate the mixture through the external circuit at a rate of 15 kg/h. Bring to boiling and total reflux, and then switch the microwave source on.j0123] Add 68.40 g acetophenone over one hour. Once it has all been added, let the reaction continue for another 15 minutes. Throughout all this time, draw the methanol off the reaction mixture. Afier the 15 minutes of finishing time, switch off the microwave source and the heatet AcidiFy the mixture and then wash it.j0124] Analysis of the organic phase by gas phase chromatography shows that almost all the acetophenone is consumed and that the DBM titre is 99.7%. DBM productivity during the reaction phase is 101.8 kg/hIm3.
References:
US2014/88325,2014,A1 Location in patent:Paragraph 0121; 0122; 0123; 0124
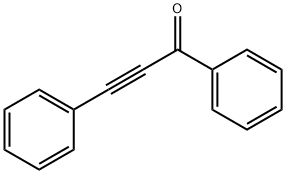
7338-94-5
40 suppliers
$220.00/100mg

120-46-7
425 suppliers
$6.00/25g
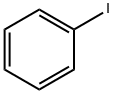
591-50-4
505 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry
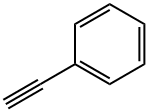
536-74-3
313 suppliers
$10.00/1g

120-46-7
425 suppliers
$6.00/25g
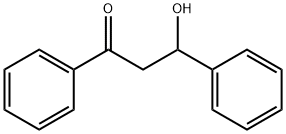
42052-51-7
0 suppliers
inquiry

120-46-7
425 suppliers
$6.00/25g