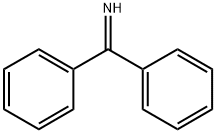
Benzophenone imine synthesis
- Product Name:Benzophenone imine
- CAS Number:1013-88-3
- Molecular formula:C13H11N
- Molecular Weight:181.23
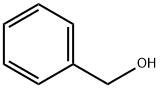
100-51-6
1431 suppliers
$5.00/100g

1013-88-3
388 suppliers
$10.00/5g
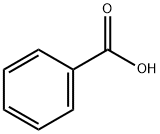
65-85-0
1400 suppliers
$10.00/25g
Yield:1013-88-3 13%
Reaction Conditions:
with oxygen in acetonitrile at 20; for 5 h;Catalytic behavior;
Steps:
2.8. General procedure for the one-pot preparation of carboxylicacid through oxidation of alcohols using Fe3O4(at)SiO2(at)Im[Cl]Mn(III)complex nanocomposite in the presence of molecular O2
General procedure: In a 10mL round bottom flask equipped with O2 purging,alcohol (10.0 mmol) and the catalyst (20 mg, 0.25 mol % Mn) wereadded to 3mL dry acetonitrile. Oxygen with flow rate of 120 mL/minwas purged to the mixture during the reaction and the reactionwas stirred at room temperature. Reaction progress was monitoredby GC. After completion of the reaction, the purge system wasremoved and the catalyst 9 was filter off by an external magnet. Theresultant mixture was washed with diethyl ether to remove ofalcohol residues. The aqueous layer was acidified with HCl (3N),then extracted with ethyl acetate. The organic layer was dried overMg2SO4, the solutionwas filtered and the desire carboxylic acidwasobtained after remove of solvent under reduced pressure. All carboxylicacids were identified by comparison of their NMR spectrawith the literature data.
References:
Kazemnejadi, Milad;Alavi, Seyyedeh Ameneh;Rezazadeh, Zinat;Nasseri, Mohammad Ali;Allahresani, Ali;Esmaeilpour, Mohsen [Journal of Molecular Structure,2019,vol. 1186,p. 230 - 249]
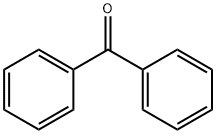
119-61-9
806 suppliers
$5.00/10g

1013-88-3
388 suppliers
$10.00/5g
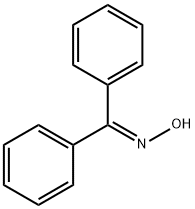
574-66-3
143 suppliers
$16.00/5g

1013-88-3
388 suppliers
$10.00/5g

108-86-1
519 suppliers
$10.00/5g
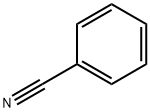
100-47-0
375 suppliers
$14.14/25gm:

1013-88-3
388 suppliers
$10.00/5g
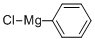
100-59-4
267 suppliers
$42.00/0.25mole

100-47-0
375 suppliers
$14.14/25gm:

1013-88-3
388 suppliers
$10.00/5g