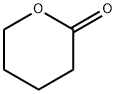
delta-Valerolactone synthesis
- Product Name:delta-Valerolactone
- CAS Number:542-28-9
- Molecular formula:C5H8O2
- Molecular Weight:100.12
120-92-3
425 suppliers
$11.00/25g

542-28-9
331 suppliers
$5.00/5g
Yield:542-28-9 99%
Reaction Conditions:
with dihydrogen peroxide;lithium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate in water;1,2-dichloro-ethane at 70; for 2 h;Time;Temperature;Reagent/catalyst;
Steps:
27
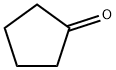
In Examples 24 to 30, as shown in Chem. 1, lactones were synthesized from various cyclic ketones by a Baeyer-Villiger oxidation reaction using hydrogen peroxide. In Example 24, when 2-methylcyclohexanone, which was an asymmetric cyclic ketone, was used, a corresponding ε-caprolactone was obtained at a high yield with normal regioselectivity. In Example 25, when 4-isopropenylcyclohexanone, which was a cyclic ketone having a substituent with an olefinic bond, was used, a corresponding ε-caprolactone was obtained at a yield of 56%, and no epoxidation of the olefin was observed. In Example 26, when 4-hydroxycyclohexanone was used, a five-membered lactone having a hydroxyethyl group was obtained. In this case, it is construed that after a corresponding ε-caprolactone was formed, this lactone was obtained by rearrangement therefrom into a five-membered ring having a smaller ring strain. In Examples 27 to 29, when a five-membered ring ketone and a four-membered ring ketone were used, corresponding six-membered ring lactone and five-membered ring lactone were obtained at a high yield. In Example 30, when a condensed ring ketone having an olefinic bond inside the ring was used, a corresponding condensed ring lactone was obtained at a high yield with normal regioselectivity, and no epoxidation of the olefin was observed.In addition, commercially available products were used for 2-methylcyclohexanone of Example 24 and cyclopentanone of Example 27, and starting raw materials of the other Examples were synthesized in accordance with the methods described in the literatures (*1 to 5 shown in Chem. 1).
References:
US2013/217898,2013,A1 Location in patent:Paragraph 0054; 0055; 0061
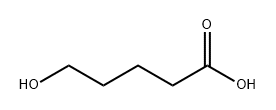
13392-69-3
40 suppliers
$135.00/1g

542-28-9
331 suppliers
$5.00/5g
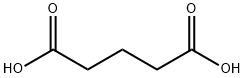
110-94-1
522 suppliers
$5.00/25g

542-28-9
331 suppliers
$5.00/5g

111-29-5
395 suppliers
$5.00/25g

542-28-9
331 suppliers
$5.00/5g
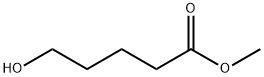
14273-92-8
53 suppliers
$165.00/100mg

542-28-9
331 suppliers
$5.00/5g