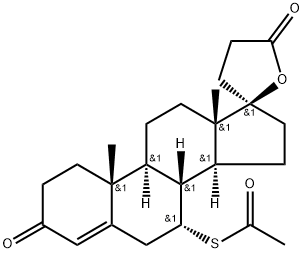
Spironolactone synthesis
- Product Name:Spironolactone
- CAS Number:52-01-7
- Molecular formula:C24H32O4S
- Molecular Weight:416.58
976-71-6
265 suppliers
$33.00/25mg
507-09-5
324 suppliers
$12.00/5g

52-01-7
494 suppliers
$17.00/1mg
Yield:52-01-7 76%
Reaction Conditions:
Stage #1: tiolacetic acidwith trifluoromethylsulfonic anhydride in tetrahydrofuran;
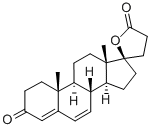
Stage #2: canrenone in tetrahydrofuran at 20; for 1 h;
Steps:
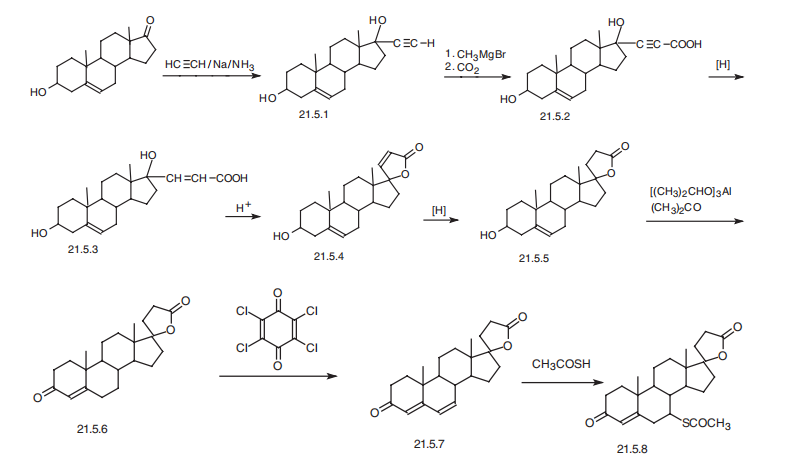
5 Example 5 Synthesis of Spiro Lactone of Compound of Formula (I)
20 mmol of freshly distilled thioacetic acid, 20 mmol of trimethylsilyl triflate and 100 mL of tetrahydrofuran were added to the reaction flask. After stirring well, 10 mmol of the compound of formula (IV) was added and the reaction was stirred at room temperature for 1 h.Then, 50 mL of ethyl acetate was added to the reaction flask,Then slowly add 50mL saturated sodium carbonate solution, stirred at room temperature for 0.5h,The reaction solution was then extracted with 50 mL of ethyl acetate in three portions, and the organic layers were combined.Drying over anhydrous sodium sulfate and evaporation of the solvent under reduced pressure gave a light brown solid,Recrystallization from methanol gave crystals of the white compound of formula (I). Yield:76%, by HPLC normalized purity: 99.6%.
References:
CN107312060,2017,A Location in patent:Paragraph 0031; 0032

976-71-6
265 suppliers
$33.00/25mg

507-09-5
324 suppliers
$12.00/5g
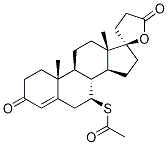
33784-05-3
40 suppliers
$160.00/1mg

52-01-7
494 suppliers
$17.00/1mg
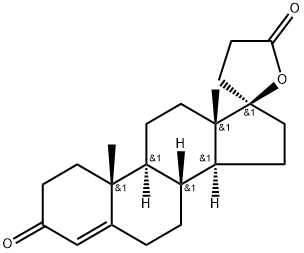
976-70-5
32 suppliers
$180.00/10mg

52-01-7
494 suppliers
$17.00/1mg
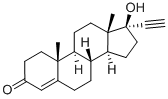
434-03-7
325 suppliers
$22.39/1G

52-01-7
494 suppliers
$17.00/1mg
![(8R,9S,10R,13S,14S,17R)-17-ethynyl-10,13-dimethyl-1,2,4,7,8,9,10,11,12,13,14,15,16,17,-tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2′-[1,3]dioxolan]-17-ol](/CAS/20211123/GIF/50407-76-6.gif)
50407-76-6
1 suppliers
inquiry

52-01-7
494 suppliers
$17.00/1mg