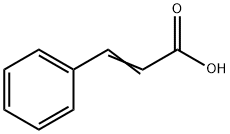
Cinnamic acid synthesis
- Product Name:Cinnamic acid
- CAS Number:621-82-9
- Molecular formula:C9H8O2
- Molecular Weight:148.16

108-86-1
513 suppliers
$10.00/5g
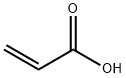
79-10-7
715 suppliers
$20.50/500ml

621-82-9
361 suppliers
$29.12/100G
Yield:621-82-9 98%
Reaction Conditions:
Stage #1: acrylic acidwith potassium tert-butylate in water at 20; for 0.166667 h;Inert atmosphere;
Stage #2: bromobenzenewith C16H21Br2N3Pd in water at 120; for 24 h;Inert atmosphere;Sealed tube;Mizoroki-Heck reaction;
Steps:
4.2.1. General procedure for the NHC-Pd(II) complex 1-catalyzed Mizoroki-Heck reaction
General procedure: (If olefin is acrylic acid) Under N2 atmosphere, acrylic acid 3a (1.5 mmol), KOtBu (3.0 equiv) and H2O (2.0 mL) were added into a seal tube and the mixture was stirred at room temperature for 10 min. Then NHC-Pd(II) complex 1 (1.0 mol %) and aryl halides 2 (1.0 mmol) were added. The mixture was stirred vigorously at 120 °C (for bromides) or 100 °C (for iodides) for 24 h. After cooling to room temperature, HCl (4 M) was dropped into the reaction mixture to reach a pH of 1, extracted with EtOAc, washed with brine, dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by a flash column chromatography (SiO2) to give the pure product.
References:
Tang, Yi-Qiang;Chu, Chun-Yan;Zhu, Lei;Qian, Bin;Shao, Li-Xiong [Tetrahedron,2011,vol. 67,# 49,p. 9479 - 9483] Location in patent:experimental part
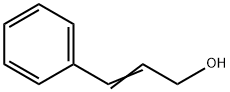
104-54-1
636 suppliers
$13.00/25g

621-82-9
361 suppliers
$29.12/100G

124-38-9
130 suppliers
$214.00/14L
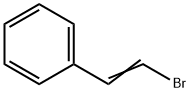
103-64-0
217 suppliers
$7.00/5g

621-82-9
361 suppliers
$29.12/100G
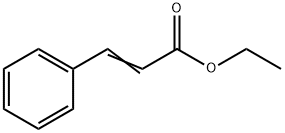
103-36-6
516 suppliers
$6.00/25g

621-82-9
361 suppliers
$29.12/100G
![[(Z)-2-bromoethenyl]benzene](/CAS/GIF/588-72-7.gif)