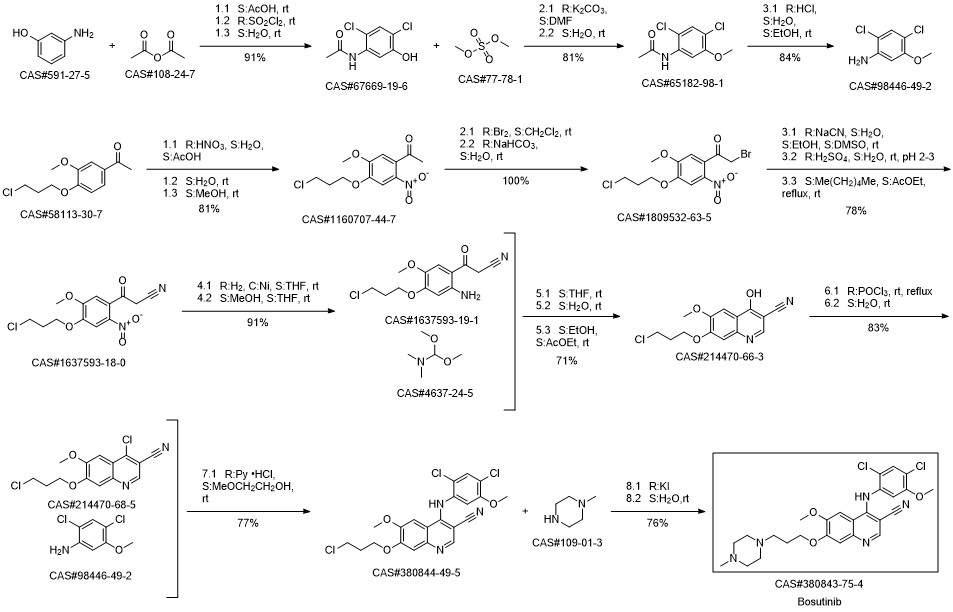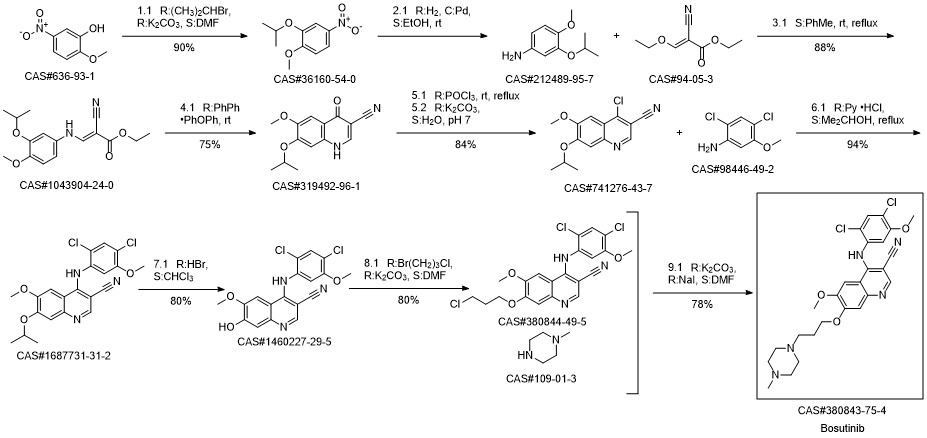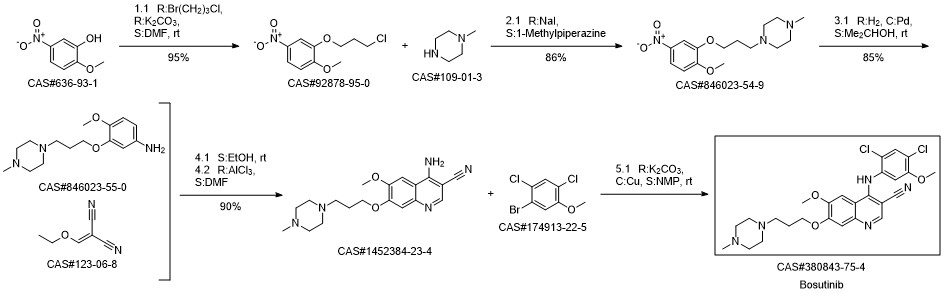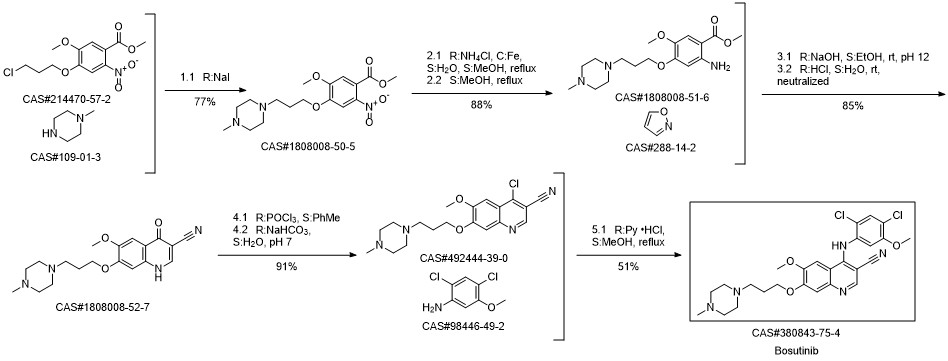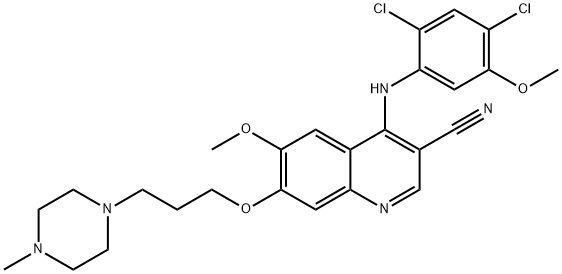
Bosutinib synthesis
- Product Name:Bosutinib
- CAS Number:380843-75-4
- Molecular formula:C26H29Cl2N5O3
- Molecular Weight:530.45
Boschelli, Diane H.; Wang, Yanong D.; Johnson, Steve; Wu, Biqi; Ye, Fei; Sosa, Ana Carolina Barrios; Golas, Jennifer M.; Boschelli, Frank. 7-Alkoxy-4-phenylamino-3-quinolinecarbonitriles as Dual Inhibitors of Src and Abl Kinases. Journal of Medicinal Chemistry. Volume 47. Issue 7. Pages 1599-1601. Journal. (2004).
109-01-3
677 suppliers
$5.00/5 g
846023-24-3
124 suppliers
inquiry
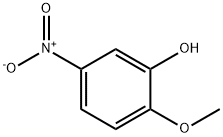
636-93-1
273 suppliers
$6.00/5g

109-70-6
495 suppliers
$10.00/5g
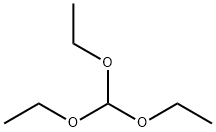
122-51-0
506 suppliers
$10.00/5ml

380843-75-4
353 suppliers
$35.00/5mg
Yield:380843-75-4 75.4%
Reaction Conditions:
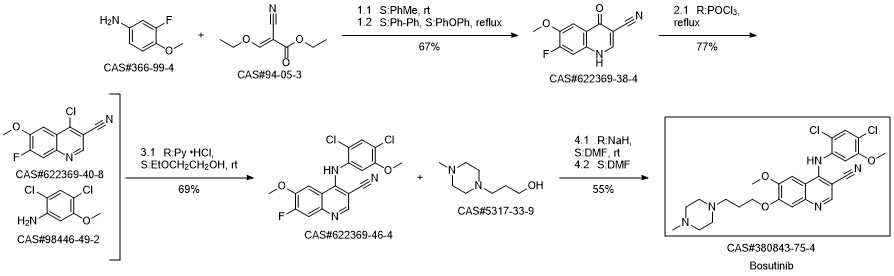
Stage #1:5-nitroguaiacol;1.3-chlorobromopropane with potassium carbonate in isopropyl alcohol at 75 - 80; for 6 h;
Stage #2:1-methyl-piperazine in isopropyl alcohol at 70 - 75; for 12 h;
Stage #3:2-cyano-N-(2,4-dichloro-5-methoxyphenyl)acetamide;orthoformic acid triethyl esterFurther stages;Solvent;Temperature;Reagent/catalyst;
Steps:
1-19 Example 1
Add SM-1 (33.83g, 0.20mol), potassium carbonate (55.28g, 0.40mol), 1-bromo-3-chloropropane (56.68g, 0.36mol) to isopropanol (350mL), and control the temperature to 75 React at 80 for 6h,After the reaction is completed, filter, the obtained filtrate is cooled to room temperature, N-methylpiperazine (40.07g, 0.40mol) is added, and the temperature is controlled at 7075 to react for 12h.After the reaction is complete, filter,The obtained filtrate was cooled to room temperature, Pd/C (3.38g) was added, hydrogen gas was introduced at normal pressure, and the temperature was controlled at 30-35°C to react for 6 hours.After the reaction is complete, filter, the obtained filtrate is cooled to room temperature, triethyl orthoformate (62.24g, 0.42mol), compound 1 (46.46 g, 0.18mol) is added, and the temperature is controlled at 7782 to react for 6h.After the reaction was completed, the reaction solution was cooled to room temperature, acetone (1400 mL) was added, and stirring was continued for 2 hours, and then suction filtered. The filter cake was rinsed with acetone (50 mL).The filter cake obtained after vacuum drying under reduced pressure is the target product II with a yield of 75.4%.The purity is 98.90%.
References:
Lunan Pharmaceutical Group Co., Ltd.;Zhang Guimin;Li Zhenyu;Sun Xiaolei CN111646955, 2020, A Location in patent:Paragraph 0041-0078

109-01-3
677 suppliers
$5.00/5 g
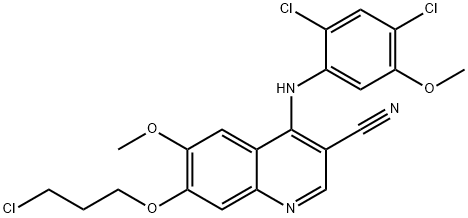
380844-49-5
97 suppliers
inquiry

380843-75-4
353 suppliers
$35.00/5mg
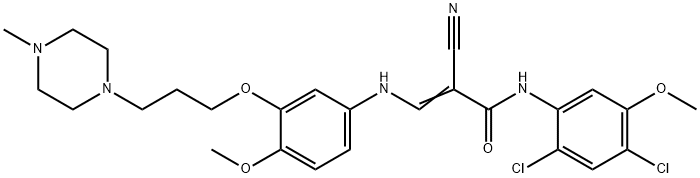
846023-56-1
12 suppliers
inquiry

380843-75-4
353 suppliers
$35.00/5mg
![3-Quinolinecarbonitrile,4-chloro-6-Methoxy-7-[3-(4-Methyl-1-piperazinyl)propoxy]-](/CAS/20150408/GIF/492444-39-0.gif)
492444-39-0
35 suppliers
inquiry
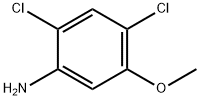
98446-49-2
264 suppliers
$6.00/1g

380843-75-4
353 suppliers
$35.00/5mg
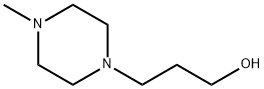
5317-33-9
138 suppliers
$10.00/1 g
![4-[(2,4-Dichloro-5-methoxyphenyl)amino]-7-fluoro-6-methoxy-3-quinolinecarbonitrile](/CAS2/GIF/622369-46-4.gif)
622369-46-4
39 suppliers
inquiry

380843-75-4
353 suppliers
$35.00/5mg