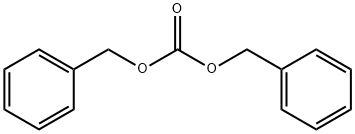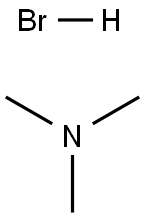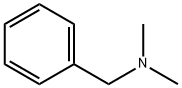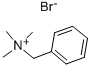
Benzyltrimethylammonium bromide synthesis
- Product Name:Benzyltrimethylammonium bromide
- CAS Number:5350-41-4
- Molecular formula:C10H16BrN
- Molecular Weight:230.14
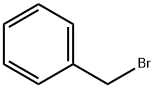
100-39-0
439 suppliers
$10.00/10 g
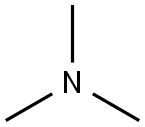
75-50-3
282 suppliers
$15.00/25ml

5350-41-4
248 suppliers
$5.00/25g
Yield:5350-41-4 98%
Reaction Conditions:
in tetrahydrofuran;ethanol at 25; for 24 h;Inert atmosphere;
Steps:
General Procedure
General procedure: In analogy to literature4, to a solution of benzyl halide 9 (1 equiv.) in THF (50 ml/g benzyl halide) were added 1.5 equiv. of trimethylamine 10 (33 wt% in EtOH).The resulting solution was stirred at RT for 24 hours, during which the product precipitated asa white solid. The resulting suspension was cooled to 0 °C, filtrated, washed with ice-cold Et2O and dried in vacuo to yield benzyl ammonium salts 6 in the reported yields.
References:
Roiser, Lukas;Robiette, Rapha?l;Waser, Mario [Synlett,2016,vol. 27,# 13,art. no. ST-2016-B0225-L,p. 1963 - 1968] Location in patent:supporting information