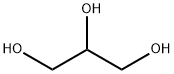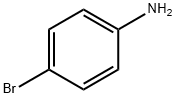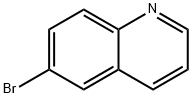
6-Bromoquinoline synthesis
- Product Name:6-Bromoquinoline
- CAS Number:5332-25-2
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
Yield:5332-25-2 81%
Reaction Conditions:
with tungstic acid functionalized KIT-6 in water at 200; for 3 h;Autoclave;Skraup Quinoline Synthesis;
Steps:
General procedure for synthesis of quinolines
General procedure: All the reactions were carried out in a 50 mL stainless steel autoclave (Scheme 1). A mixture of glycerol (1 g, 1 equiv.), aniline (0.58 g, 0.5 equiv.), water (2.5 mL) and W-KIT-6 (100 mg) was charged in to the autoclave simultaneously and finally heated to 200 °C with stirring for 3 h. On completion of the reaction (monitored by TLC), the autoclave was cooled to room temperature and the catalyst was removed by filtration. Ethyl acetate (10 mL) and water (5 mL) were added to the reaction mixture and stirred well for few minutes. The organic layer was dried over anhydrous Na2SO4 and the crude was purified by column chromatography using 60-120 mesh silica with ethylacetate/hexane as eluent to afford the desired product in good yield. The product was analyzed and confirmed with GC-MS and 1H NMR and 13C NMR spectroscopy (Table 1 and Figs S1-S5, Supplementary Data).
References:
Udayakumar;Pandurangan [Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical,2016,vol. 55A,# 8,p. 919 - 928]
22190-35-8
155 suppliers
$25.00/250mg

5332-25-2
420 suppliers
$6.00/5g
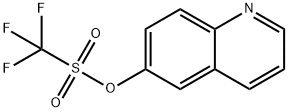
173089-80-0
27 suppliers
$80.40/5g

5332-25-2
420 suppliers
$6.00/5g
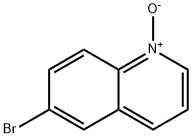
6563-11-7
12 suppliers
inquiry

5332-25-2
420 suppliers
$6.00/5g
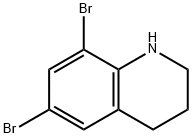
190843-73-3
13 suppliers
inquiry

5332-25-2
420 suppliers
$6.00/5g