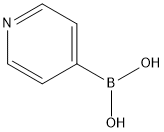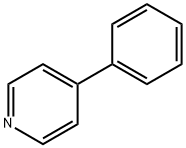
4-Phenylpyridine synthesis
- Product Name:4-Phenylpyridine
- CAS Number:939-23-1
- Molecular formula:C11H9N
- Molecular Weight:155.2
Yield:939-23-1 100%
Reaction Conditions:
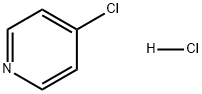
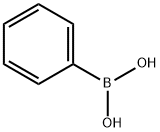
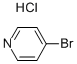
with caesium carbonate in N,N-dimethyl acetamide at 80; for 12 h;Inert atmosphere;Suzuki-Miyaura Coupling;
Steps:
Typical Procedure for the 7% Pd/WA30-Catalyzed Suzuki-Miyaura Reaction between Aryl Chlorides and Arylboronic Acids (Table 2 and Scheme 2)
General procedure: In the test tube were placed 7% Pd/WA30 (19.0 mg, 12.5 μmol), the aryl chloride (250 μmol), the arylboronic acid (375 μmol), Cs2CO3 (163 mg, 500 μmol), and DMA (1 mL). The mixture was stirred at 80 °C under an Ar atmosphere. The reaction progress was monitored by TLC analysis (hexane-EtOAc, 5:1). When the reaction was completed within 24 h, the mixture was cooled to r.t., diluted with Et2O (5 mL), and passed through a cotton filter. The catalyst on the filter was washed with Et2O (2 × 15 mL) and H2O (3 × 10 mL). The combined filtrate was separated into two layers. The aqueous layer was extracted with Et2O (20 mL), and the combined organic layers were washed with H2O (4 × 20 mL) and brine (20 mL), dried over Na2SO4, filtered, and concentrated in vacuo. To the residue was added CDCl3 (ca. 1 mL) and 1,4-dioxane (8.53 μL, 100 μmol). After the determination of the reaction yield by 1H NMR, the product was purified by silicagel column chlomatography using hexane-EtOAc (10:1) as eluents to give the corresponding biaryl. When the reaction was incomplete after 24 h, the reaction mixture was treated in the same manner as described above.
References:
Monguchi, Yasunari;Ichikawa, Tomohiro;Netsu, Moeko;Hattori, Tomohiro;Mizusaki, Tomoteru;Sawama, Yoshinari;Sajiki, Hironao [Synlett,2015,vol. 26,# 14,art. no. ST-2015-U0258-L,p. 2014 - 2018] Location in patent:supporting information
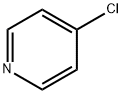
626-61-9
54 suppliers
$365.00/500mg
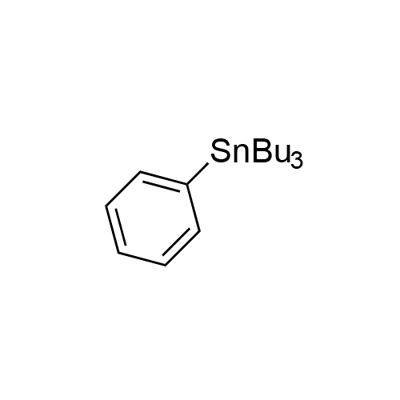
960-16-7
98 suppliers
$22.60/1g

939-23-1
218 suppliers
$10.00/1g