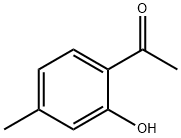
2'-Hydroxy-4'-methylacetophenone synthesis
- Product Name:2'-Hydroxy-4'-methylacetophenone
- CAS Number:6921-64-8
- Molecular formula:C9H10O2
- Molecular Weight:150.17
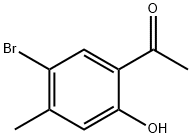
50342-17-1
43 suppliers
$34.79/1g

6921-64-8
189 suppliers
$29.00/1g
Yield:6921-64-8 96%
Reaction Conditions:
with palladium on activated charcoal;hydrogen;sodium hydroxide in methanol at 50; for 3 h;
Steps:
3.7 Synthesis of acetophenone 19
A mixture of acetophenone 15 (1.15 g, 5.0 mmol), Pd/C (0.50 g), and sodiumhydroxide (NaOH, 0.20 g, 5.0 mmol) in methanol (10 mL) in an hydrogen atmospherewas stirred at 50 °C for 3 hours. The mixture was added with brine (30 mL) and ethylacetate (30 mL), and the aqueous phase was extracted with ethyl acetate (3 x 30 mL).The combined organic phases were washed with brine (30 mL), dried over anhydroussodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure. Theresidue was purified by column chromatography (33% dichloromethane in petroleumether) silica gel to afford acetophenone 19 as a pale yellow liquid (0.72 g). Yield:96%; 1 H NMR (400 MHz, CDCl3): /ppm = 12.28 (s, 1H), 7.56 (d, J = 8.0 Hz, 1H),6.73 (s, 1H), 6.66 (dd, J = 8.0, 1.0 Hz, 1H), 2.55 (s, 3H), 2.31 (s, 3H); 13C NMR (100MHz, CDCl3): = 203.8, 162.3, 147.9, 130.5, 120.1, 118.2, 117.4, 26.2, 21.7; IRmax = 3005, 1740, 1575, 1507, 1431, 1367, 1324, 1303, 1246, 1225, 1166,1149, 1023, 977, 933, 795, 737, 695 cm-1. HRMS (ESI): m/z calcd for C9H10NaO2 [M+ Na]+: 173.0573, found: 173.0576.
References:
Gong, Pi-Xian;Li, Hui-Jing;Wang, Meirong;Cheng, Yun-Fei;Wu, Yan-Chao [Natural Product Research,2019,vol. 33,# 20,p. 2911 - 2916] Location in patent:supporting information
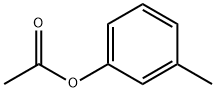
122-46-3
114 suppliers
$21.00/1g

6921-64-8
189 suppliers
$29.00/1g
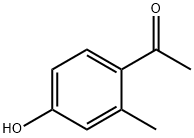
875-59-2
133 suppliers
$6.00/250mg

122-46-3
114 suppliers
$21.00/1g

6921-64-8
189 suppliers
$29.00/1g
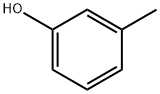
108-39-4
502 suppliers
$13.00/25g
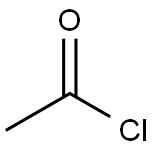
75-36-5
589 suppliers
$17.92/100G

6921-64-8
189 suppliers
$29.00/1g
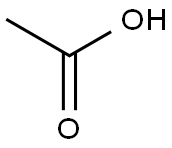
64-19-7
1584 suppliers
$10.00/25ML

108-39-4
502 suppliers
$13.00/25g

6921-64-8
189 suppliers
$29.00/1g