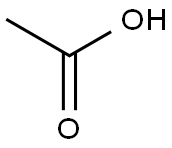
Acetic acid synthesis
- Product Name:Acetic acid
- CAS Number:64-19-7
- Molecular formula:C2H4O2
- Molecular Weight:60.05
Vinegars are produced from cider, grapes (or wine), sucrose, glucose or malt by successive alcoholic and acetous fermentations. In the United States, the use of the term “vinegar,” without qualifying adjectives, implies only cider vinegar. Although a 4 to 8% solution of pure acetic acid would have the same taste characteristics as cider vinegar, it could not qualify as a vinegar, since it would lack other readily detectable components characteristic of cider vinegar. In Great Britain, malt vinegar is specified. On the European continent, wine vinegar is the most common variety
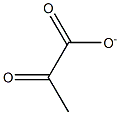
57-60-3
1 suppliers
inquiry

64-19-7
1585 suppliers
$10.00/25ML
Yield:64-19-7 100%
Reaction Conditions:
with sodium hypochlorite in aq. phosphate buffer at 0 - 20; pH=4;
Steps:
Aldehyde oxidation. Method C
General procedure: Aldehyde (70-350 mM), an internal standard (DSS or 20) and the specified additive (none, NH4Cl, H2O2, DMS, DMSO, sulfamic acid or L-methinone) were dissolved in phosphate buffer (60 mM, D2O, pH 4-7). Sodium chlorite (5 M, 1.4 equiv.) was added in five portions over 1 h at 0 °C, then the solution was warmed to ambient temperature and NMR spectra were acquired. The carboxylic acid product was confirmed by sample spiking, and the yield (Table 1 and Supplementary Table 1) was quantified with respect to the internal standard.
References:
Coggins, Adam J.;Powner, Matthew W. [Nature Chemistry,2017,vol. 9,# 4,p. 310 - 317]
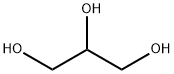
56-81-5
1703 suppliers
$5.00/25g

64-19-7
1585 suppliers
$10.00/25ML
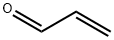
107-02-8
0 suppliers
$38.60/4s8501

67-56-1
776 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry
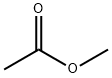
79-20-9
570 suppliers
$14.00/100g

64-19-7
1585 suppliers
$10.00/25ML

56-81-5
1703 suppliers
$5.00/25g
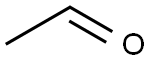
75-07-0
425 suppliers
$14.00/5mL

64-19-7
1585 suppliers
$10.00/25ML
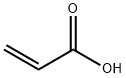
79-10-7
724 suppliers
$20.50/500ml
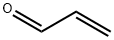
107-02-8
0 suppliers
$38.60/4s8501
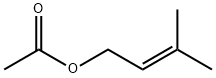
1191-16-8
204 suppliers
$10.00/25g

64-19-7
1585 suppliers
$10.00/25ML