2-Bromotoluene synthesis
- Product Name:2-Bromotoluene
- CAS Number:95-46-5
- Molecular formula:C7H7Br
- Molecular Weight:171.03
Yield:95-46-5 79%
Reaction Conditions:
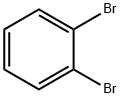
Stage #1:2,3-dibromobenzene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.000227778 h;
Stage #2:methyl trifluoromethanesulfonate in tetrahydrofuran;diethyl ether;hexane at -78; for 0.000641667 - 0.001925 h;Product distribution / selectivity;
Steps:
1; 8
Example 1; The present invention was carried out using a microreactor apparatus, as shown in FIG. 1.A microsystem composed of four T-shaped micromixers (M1, M2, M3 and M4) and four microtube reactors (R1, R2, R3 and R4) was used. The M1, R1, M2 and R2 were dipped in a cooling bath (-78° C.), and the M3, R3, M4 and R4 were dipped in a cooling bath (0° C.). A reaction solution flowed from R4 was cooled at 0° C., and was collected in a flask. A solution of o-dibromobenzene (0.27 M) in THF (flow rate: 6 mL/min, 1.62 mmol/min) and a solution of n-BuLi (1.5 M) in n-hexane (flow rate: 1.2 mL/min, 1.8 mmol/min) were introduced to the first T-shaped mixer M1 (inner diameter: 250 μm) by using syringe pumps. A solution of methyl trifluoromethanesulfonate (MeOTf) (0.65 M) in THF (flow rate: 3 mL/min, 1.95 mmol/min) was introduced to the second T-shaped mixer M2 (inner diameter: 500 μm). A solution of n-BuLi (1.5 M) in n-hexane (flow rate: 1.8 mL/min, 2.7 mmol/min) was introduced to the third T-shaped mixer M3 (inner diameter: 500 μm). A solution of chlorotrimethylsilane (1.62 M) in THF (flow rate: 3 mL/min, 4.86 mmol/min) was introduced to the fourth T-shaped mixer M4 (inner diameter: 500 μm). The residence time of the tube reactor R1 (inner diameter: 500 μm, length=50 cm) was 0.82 second. The residence time of the tube reactor R2 (inner diameter: 1,000 μm, length=150 cm) was 6.93 second. The residence time of the tube reactor R3 (inner diameter: 1,000 μm, length=12.5 cm) was 0.49 second. The residence time of the tube reactor R4 (inner diameter: 1,000 μm, length=150 cm) was 1.57 second. After passing through R4, an aliquot of the product solution was taken (15 sec) and was stirred for 1 hour in ice bath (0° C.). As a result of the product solution was analyzed by GC (column, CBP1; 0.25 mm×25 m; initial oven temperature, 50° C.; rate of temperature increase, 10° C./min), trimethyl(o-tolyl)silane (GC retention time: 13.5 min) was obtained in 67% yield. 1H NMR (400 MHz, CDCl3) δ: 0.32 (s, 9H), 2.45 (s, 3H), 7.14 (tt, J=7.6, 0.4 Hz, 2H), 7.25 (t, J=7.2 Hz, 1H), 7.44 (d, J=6.8 Hz, 1H); 13C NMR (100 MHz) δ: 0.0, 23.1, 124.7, 129.0, 129.6, 134.1, 138.2, 143.3. The thus-obtained spectral data were identical to those described in a literature. Reaction components used in this Example and the results were shown in Table 1 set forth below.; Example 8; E1=Me and X2Br in Formula (IV)The present invention was carried out using a microreactor apparatus, as shown in FIG. 3.A microsystem composed of three T-shaped micromixers (M1, M2 and M3) and two microtube reactors (R1 and R2) was used. The whole microsystem was dipped in a cooling bath (-78° C.). A solution of o-dibromobenzene (0.27 M) in THF (flow rate: 6 mL/min, 1.62 mmol/min) and a solution of n-BuLi (1.5 M) in n-hexane (flow rate: 1.2 mL/min, 1.8 mmol/min) were introduced to the first T-shaped mixer M1 (inner diameter: 250 μm) by using syringe pumps. The resulting solution was passed through the tube reactor R1 and was mixed with methyl trifluoromethanesulfonate (MeOTf) (0.65 M) in Et2O (flow rate: 3 mL/min, 1.95 mmol/min) in the second T-shaped mixer M2 (inner diameter: 500 μm). The resulting solution was passed through the tube reactor R2 and was introduced to the third T-shaped mixer M3 (inner diameter: 500 μm), where the solution was quenched with methanol (neat, flow rate=3.0 mmol/min). The residence time of the tube reactor R1 (inner diameter: 500 μm, length=50 cm) was 0.82 second. The residence time of the tube reactor R2 (inner diameter: 1,000 μm, length=50 cm) was 2.31 second. After finishing the reaction, an aliquot of the product solution was taken (15 sec) and was analyzed by GC (column, CBP1; 0.25 mm×25 m; initial oven temperature, 50° C.; rate of temperature increase, 10° C./min). As a result, o-bromotoluene (GC retention time: 11.5 min) was obtained in 79% yield.
References:
FUJIFILM Corporation US2008/194816, 2008, A1 Location in patent:Page/Page column 13; 15; 17

108-88-3
0 suppliers
$21.80/5 mL

95-46-5
307 suppliers
$10.00/10 g
103-73-1
355 suppliers
$8.00/25g
106-38-7
413 suppliers
$14.00/5g

95-46-5
307 suppliers
$10.00/10 g
31543-75-6
182 suppliers
$12.10/1g
100-39-0
439 suppliers
$10.00/10 g
2093-46-1
2 suppliers
inquiry

95-46-5
307 suppliers
$10.00/10 g