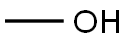Methyl trifluoromethanesulfonate synthesis
- Product Name:Methyl trifluoromethanesulfonate
- CAS Number:333-27-7
- Molecular formula:C2H3F3O3S
- Molecular Weight:164.1
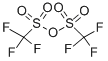
CF3SO2OH + (CH3O)2SO2 → CF3SO2OCH3 + CH3OSO2OH

111-96-6
505 suppliers
$16.00/25mL
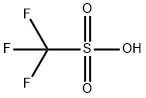
1493-13-6
632 suppliers
$12.00/5g

333-27-7
303 suppliers
$12.00/1g
Yield:333-27-7 95.6 %
Reaction Conditions:
with phosphorus pentoxide at -5 - 100;Inert atmosphere;Sealed tube;
Steps:
7 [Example 7]
In a space of a nitrogen atmosphere dried with a dry column (manufactured by Nikka Seiko Co., Ltd., DC-A4),diphosphorous pentoxide (20.9 g, 147 mmol) in a flask,Diethylene glycol dimethyl ether (35.9 g, 268 mmol) charged with half-moon stir bar with stir seal,A Liebig condenser with a receiver, a three-way cock, and a dropping funnel containing trifluoromethanesulfonic acid were attached and removed from the nitrogen atmosphere space.Dry nitrogen was introduced from the three-way cock, a -5° C. refrigerant was passed through the Liebig condenser, and the receiver attached to the Liebig condenser was cooled with ethanol cooled with dry ice.While stirring, trifluoromethanesulfonic acid was gradually added dropwise. After dropping was completed, the dropping funnel and three-way cock were removed, a glass stopper was attached, and the mixture was stirred for 20 minutes.At this time, 20.1 g (134 mmol) of trifluoromethanesulfonic acid was introduced into the flask.The flask was placed in an oil bath (OHB-3100S, manufactured by Tokyo Rikakikai Co., Ltd.) set at 60° C. and stirred for 20 minutes.The oil bath was set to 100° C., and after stirring for 20 minutes, the pressure was gradually reduced to 70 kPaA, and the distillate was recovered in a receiver.After confirming that no more distillation had occurred, the stirring was stopped.After purging the flask with nitrogen, it was removed from the oil bath, the flow of refrigerant in the Liebig condenser was stopped, and the receiver was removed from the ethanol cooled with dry ice and returned to room temperature.As a result of measuring the mass of the contents of the receiver, it was 21.2 g.The contents of the receiver (0.30 g), hexafluorobenzene (1.30 g) and benzotrifluoride (0.10 g) were mixed and analyzed by 19F-NMR.As a result of analysis, it was found that 21.0 g (128 mmol, yield 95.6%) of a fluorine-containing sulfonate ester (10) represented by the following general formula (10) was obtained.
References:
JP2022/141493,2022,A Location in patent:Paragraph 0070
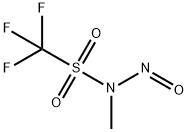
114533-01-6
0 suppliers
inquiry

333-27-7
303 suppliers
$12.00/1g