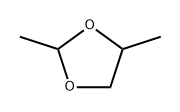
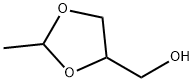
2,4-DIMETHYL, 1,3-DIOXALONE synthesis
- Product Name:2,4-DIMETHYL, 1,3-DIOXALONE
- CAS Number:3390-12-3
- Molecular formula:C5H10O2
- Molecular Weight:102.13
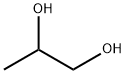
57-55-6
1560 suppliers
$5.00/1g

3390-12-3
72 suppliers
$60.00/2 g
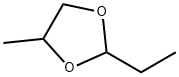
4359-46-0
25 suppliers
$74.23/5gm:

124-38-9
129 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry
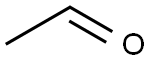
75-07-0
422 suppliers
$14.00/5mL
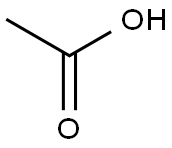
64-19-7
1563 suppliers
$10.00/25ML
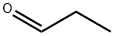
123-38-6
443 suppliers
$14.00/5mL
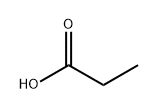
802294-64-0
1 suppliers
inquiry
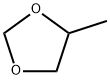
1072-47-5
61 suppliers
$50.00/1ml
Yield:-
Reaction Conditions:
with oxygen in water at 220; for 1 h;Flow reactor;Temperature;Reagent/catalyst;
Steps:
2.3. Catalytic tests
Gas-phase reactivity experiments were performed using a continuousflow reactor made of glass. A catalyst amount ranging from 0,1 to 0,5 g wasloaded in powder form. The time factor was calculated as the ratio betweencatalyst mass (g) and total gas flow (mL/s), the latter being measured atroom temperature. It was varied by keeping constant the total gas flow andchanging the catalyst amount. Inlet feed composition was also changedaccording to the desired compositions. If not differently specified, thecatalytic results were obtained after a reaction time of 60 min. The effluent stream was bubbled through three in-series devicesfilled with water, kept at 0–2 °C, for the abatment of condensable organiccompounds. After the abatement step, the gaseous stream stillcontaining oxygen and carbon oxides, was fed into an automatic samplingsystem for on-line gas chromatography (GC-TCD) analysis. Theaqueous solution was analyzed off-line by GC-FID analysis, using a referencestandard (valeric acid). Both gas chromatographic analyseswere performed with a Hewlett–Packard 5890 instrument, equippedwith either FI and TC detectors. A semi-capillary ZB-FFAP (nitroterephthalicacid modified polyethylene glycol) column was used for theseparation of condensed compounds, whereas two wide-bore columnswere used for the separation of non-condensable products: a Mol Sieve5A Plot for O2 and CO, and a Silica Plot for CO2. Compounds wereidentified by means of both GC–MS analysis and the injection of purereference standards for the comparison of retention times. Conversion,yields and selectivities were calculated by the following formulas:
References:
Bandinelli, Claudia;Basile, Francesco;Cavani, Fabrizio;Concepcion, Patricia;De Maron, Jacopo;Dimitratos, Nikolaos;Lambiase, Barbara;Nieto, Jose Manuel Lopez;Tabanelli, Tommaso [Applied Catalysis A: General,2019]
56-81-5
1663 suppliers
$5.00/25g

3390-12-3
72 suppliers
$60.00/2 g
3773-93-1
7 suppliers
inquiry
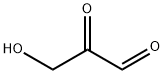
997-10-4
13 suppliers
inquiry
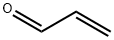
107-02-8
4 suppliers
$38.60/4s8501

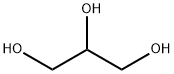
57-55-6
1560 suppliers
$5.00/1g

74-86-2
73 suppliers
inquiry

3390-12-3
72 suppliers
$60.00/2 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry