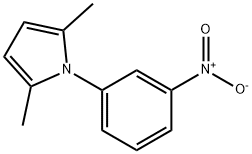
1-(3-NITROPHENYL)-2,5-DIMETHYLPYRROLE synthesis
- Product Name:1-(3-NITROPHENYL)-2,5-DIMETHYLPYRROLE
- CAS Number:32570-23-3
- Molecular formula:C12H12N2O2
- Molecular Weight:216.24
Yield:32570-23-3 100%
Reaction Conditions:
Stage #1: 3-nitro-anilinewith toluene-4-sulfonic acid in toluene; for 0.0833333 h;Dean-Stark;
Stage #2: 2,5-hexanedione for 1.25 h;Inert atmosphere;Reflux;Dean-Stark;
Steps:
9 4.2.9 2,5-Dimethyl-1-(3-nitrophenyl)-1H-pyrrole (9)
In a Dean-Stark setup a solution of 3-nitroaniline (8) (13.9 g, 100 mmol), a catalytic amount of p-toluenesulfonic acid monohydrate (0.29 g, 1.5 mmol) in toluene (350 mL) was stirred at elevated temperature.
After 5 min hexane-2,5-dione (12.5 mL, 107 mmol) was added via an addition funnel and refluxed under nitrogen atmosphere for 75 min.
After cooling the reaction was quenched with sat. aq. NaHCO3 solution (100 mL) and separated.
The organic layer was washed with water (100 mL), 0.1 M NaOH (2* 50 mL), 0.1 M HCl (2 * 50 mL), water (50 mL) and brine (50 mL).
The organic fraction was collected, dried over anhydrous magnesium sulfate, filtrated and evaporated to dryness under reduced pressure.
On cooling the brown oil crystallized to obtain the title compound (21.7 g, 100 mmol, 100%). Rf 0.84 (ethyl acetate/hexanes, 33/66, v/v).
1H NMR (CDCl3) δ 8.28 (ddd, J = 8.12 Hz, 2.22 Hz, 1.21 Hz, 1H, HAryl), 8.12 (t, J = 2.01 Hz, 1H, HAryl), 7.68 (t, J = 7.92 Hz, 1H, HAryl), 7.60-7.56 (m, 1H, HAryl), 5.95 (s, 2H, pyrrole), 2.07 (s, 6H, CH3).
13C NMR (CDCl3) δ 148.80 (Ar-NO2), 140.35 (Ar-pyrrole), 134.38 (Ar), 130.07 (Ar), 128.75 (pyrrole-CH3), 123.39 (Ar), 122.62 (Ar), 107.11 (pyrrole-CH), 13.15 (CH3).
References:
Klein, Pieter J.;Chomet, Marion;Metaxas, Athanasios;Christiaans, Johannes A.M.;Kooijman, Esther;Schuit, Robert C.;Lammertsma, Adriaan A.;Van Berckel, Bart N.M.;Windhorst, Albert D. [European Journal of Medicinal Chemistry,2016,vol. 118,p. 143 - 160]
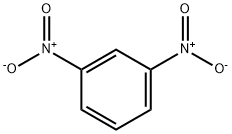
99-65-0
10 suppliers
$32.00/25g
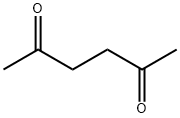
110-13-4
386 suppliers
$10.00/5g

32570-23-3
17 suppliers
$28.60/50MG
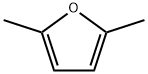
625-86-5
200 suppliers
$20.00/25mL
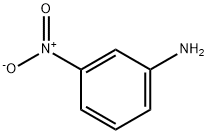
99-09-2
13 suppliers
$14.00/1g

32570-23-3
17 suppliers
$28.60/50MG