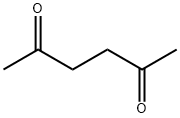
2,5-Hexanedione synthesis
- Product Name:2,5-Hexanedione
- CAS Number:110-13-4
- Molecular formula:C6H10O2
- Molecular Weight:114.14
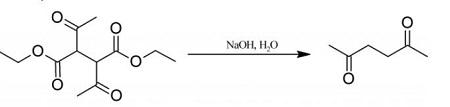
20 g of diethyl 2,3-diacetylbutanedioate are mechanically shaken for several days with 250 ml (excess) of 5% aqueous sodium hydroxide, and until no diethyl 2,3-diacetylbutanedioate separates on acidification of a sample with dilute hydrochloric acid. The solution is then saturated with potassium carbonate and extracted with ether, the extract is washed with brine to remove alcohol, dried over calcium chloride, and distilled, the fraction 192-198° C being retained. You can get 2,5-Hexanedione with a yield of 70%. Colourless liquid; agreeable odour; miscible with water, alcohol and ether; m.p. 9° C; b.p. 194° C; d=0.973 g/ml at 20° C.
625-86-5
208 suppliers
$20.00/25mL

110-13-4
404 suppliers
$10.00/5g
Yield:110-13-4 95%
Reaction Conditions:
with carbon dioxide in lithium hydroxide monohydrate at 150; under 30003 Torr; for 15 h;Autoclave;
Steps:
3 Example 3 Preparation of HDX from DMF using CO2/H2O catalyst
Example 3 Preparation of HDX from DMF using CO2/H2O catalyst (0128) A 5ml water solution of DMF (150mg, 1.56mmol) was placed inside an autoclave and CO2 was introduced, to reach a pressure of 40 bar. Under this pressure, the reaction mixture was stirred and heated to 150°C, for 15 hours. The reaction mixture was then let cool to room temperature, after which the reactor was vented and opened to release CO2. The thus obtained aqueous mixture was analysed by GC using biphenyl as the internal standard. The DMF conversion was 100%, and the yield of HDX was as high as 95%
References:
WO2015/127662,2015,A1 Location in patent:Paragraph 0075
620-02-0
497 suppliers
$14.14/5gm:

110-13-4
404 suppliers
$10.00/5g
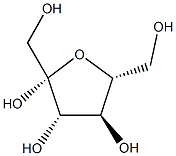
470-23-5
11 suppliers
inquiry

110-13-4
404 suppliers
$10.00/5g
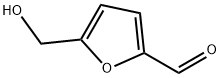
67-47-0
609 suppliers
$5.00/100mg
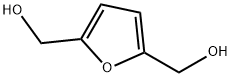
1883-75-6
284 suppliers
$8.00/250mg
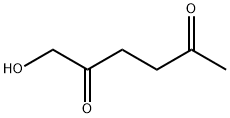
65313-46-4
2 suppliers
inquiry

110-13-4
404 suppliers
$10.00/5g