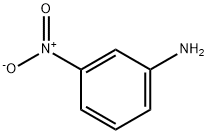
3-Nitroaniline synthesis
- Product Name:3-Nitroaniline
- CAS Number:99-09-2
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
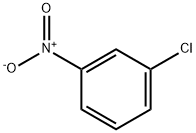
121-73-3
42 suppliers
$10.00/1g

99-09-2
20 suppliers
$14.00/1g
Yield:99-09-2 98 %Chromat.
Reaction Conditions:
with ammonium hydroxide;potassium phosphate;Cu0.18Bi0.88O0.88I1.06 in ethanol;water at 80; for 20 h;Inert atmosphere;Green chemistry;
Steps:
2.3. Catalytic procedure for the Ullmann-type CN- coupling reactions
General procedure: For testing the catalytic capabilities of oxohalides, Ullmann-type CN- coupling reactions were carried out. The mixtures of CuI (0.01-0.1 mmol) or CuIBiOI (0.01-0.1 mmol for Cu) or BiOI (0.01-0.1 mmol for Bi) catalyst, organic additive (0.05 mmol, OH-l-proline only for CuI), (hetero)aryl halide (0.5 mmol), base (1.0 mmol) and aqueous ammonia (1.0 mmol) as well as 3.0 ml of solvent were stirred in a 10 ml flask in N2 atmosphere for 1-24 h, at 25-110 °C. After cooling to room temperature, the crude product was diluted with ethyl acetate (~40 ml). The organic phase was separated and the aqueous phase was extracted with ethyl acetate twice. The combined organic phase was dried over Na2SO4. The product was purified by silica gel chromatography using solvent mixtures (ethyl acetate/hexanes, diethyl ether/hexanes). (0017) At the end of the reactions, the mixtures were analyzed on a Hewlett-Packard 5890 Series II gas chromatograph equipped with flame ionization detector using an Agilent HP-1 column and the internal standard technique using toluene. The temperature was increased in stages from 50 °C to 300 °C. The products were identified via using authentic samples.
References:
Djerdj, Igor;Kónya, Zoltán;Kocsis, Marianna;Kukovecz, ákos;Pálinkó, István;Sipos, Pál;Varga, Gábor [Molecular catalysis,2020,vol. 493]
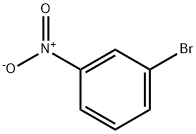
585-79-5
15 suppliers
$15.00/1g

99-09-2
20 suppliers
$14.00/1g
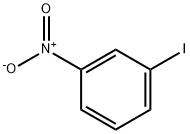
645-00-1
12 suppliers
$45.39/25g

99-09-2
20 suppliers
$14.00/1g
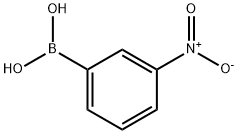
13331-27-6
356 suppliers
$5.00/1g

99-09-2
20 suppliers
$14.00/1g
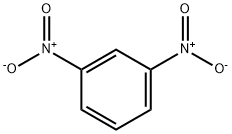
99-65-0
15 suppliers
$31.39/442244

99-09-2
20 suppliers
$14.00/1g