Sodium triacetoxyborohydride
|
|
Sodium triacetoxyborohydride ??
- ???
- 116-120 °C (dec.) (lit.)
- ?? ?
- 111.1℃[at 101 325 Pa]
- ??
- 1.36[at 20℃]
- ???
- 0Pa at 25℃
- ?? ??
- Inert atmosphere,Room Temperature
- ???
- ??? ????, ???, ??, ???, ???????, ??? ? ?????? ?????.
- ??? ??
- ??
- ??
- ???
- ???
- ?? ??
- ??
- Moisture Sensitive
- Merck
- 14,8695
- BRN
- 4047608
- InChIKey
- HHYFEYBWNZJVFQ-UHFFFAOYSA-N
- LogP
- -2.88--1.09 at 20℃
- CAS ??????
- 56553-60-7(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,Xi,C | ||
|---|---|---|---|
| ?? ???? ?? | 15-34-14/15-37/38-11 | ||
| ????? | 43-7/8-45-36/37/39-26 | ||
| ????(UN No.) | UN 1409 4.3/PG 2 | ||
| WGK ?? | 3 | ||
| F ?????? | 10-21 | ||
| ?? ?? ?? | Irritant/Flammable | ||
| TSCA | Yes | ||
| ?? ?? | 4.3 | ||
| ???? | III | ||
| HS ?? | 29319090 |
Sodium triacetoxyborohydride C??? ??, ??, ??
??? ??
Sodium triacetoxyborohydride (STAB-H) is commercially available as a hygroscopic white powder with a melting point of 116-120 °C. freely soluble in benzene. It is prepared by the reaction of NaBH4 with excess acetic acid in benzene or toluene.??
Sodium Triacetoxyborohydride(STAB) is a hydride reagent used in stereoselective reductive amination. It is able to replace toxic sodium cyanoborohydride under most conditions. It is selective in reducing aldehydes to alcohols in the presence of ketones. STAB is also stable in anhydrous acids, which enables reductive amination of aldehydes and ketones. It used in reductive amination of ketones and aldehydes and reductive amination/lactamization of carbonyl compounds with amines. The advantage of STAB compared to sodium cyanoborohydride is evident. STAB, being non-toxic, is easier to handle and forms no toxic by-products, making the treatment of process waste after the reaction simple and less costly.?? ??
Sodium triacetoxyborohydride is a mild reagent that exhibits remarkable selectivity as a reducing agent. It reduces aldehydes but not ketones; however, beta-hydroxyketones can be reduced selectively to give 1,3-trans diols.The steric and the electron-withdrawing effects of the three acetoxy groups stabilize the boron-hydrogen bond and are responsible for its mild reducing properties.Sodium triacetoxyborohydride or [NaBH(OAc)3] can be used as a reagent:
In the reductive amination of ketones and aldehydes.
To prepare N-benzyl-γ-valerolactam by reacting with methyl 4-oxopentanoate and benzylamine via reductive amination/lactamization.
To reduce imines and enamines to corresponding amines.
To reduce quinolines and isoquinolines to corresponding tetrahydro derivatives.
In the hydroboration of alkenes.
To synthesize nitroxide biradicals for creating high relaxivity terminal groups linkage to dendrimers.
?? ??
Sodium Triacetoxyborohydride is selective reducing agent in organic synthesis. It is especially suitable for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound. The presence of a stoichiometric amount of acetic acid, which catalyzes the imine formation and provides the iminium ion, doesn't present any problem under these conditions.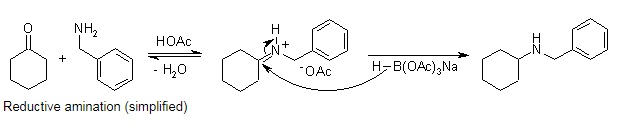
Reductive amination (simplified)
Sodium triacetoxyborohydride ?? ?? ? ???
???
?? ??
Sodium triacetoxyborohydride ?? ??
???( 695)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shandong Believe Chemical PTE.LTD | +86-18553607090 +8618553607090 |
1093908296@qq.com | China | 1667 | 58 |
| Wuhan wingroup Pharmaceutical Co., Ltd | +86-13296627870; +8613296627870 |
info@whwingroup.com | China | 695 | 58 |
| Wuhan Mayue Longteng Technology Development Co., Ltd. | +undefined15171477138 |
helen.yan@lantu-tech.com | China | 300 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7494 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 |
Ethan@dornechem.com | China | 3102 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18154 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 2990 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 997 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |










