Bornitrid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37:Reizt die Augen und die Atmungsorgane.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
Boron nitride is a material in which the extra electron of nitrogen (with respect to carbon) enables it to form structures that are isoelectronic with carbon allotropes.
Boron nitride is an inorganic compound with a flat, hexagonal crystal similar to graphite, but with the carbon atoms replaced by boron and nitrogen atoms. The alternate boron and nitrogen atoms are linked to form interlocking hexagonal rings with three boron atoms and three nitrogen atoms, and the layers are held together by van der Waals forces. There is no boron-nitrogen bonding between the layers.The bond length is 1.466Å and the interlayer spacing is 3.331 Å. A spherical form (with a hexagonal crystal structure) is also available.
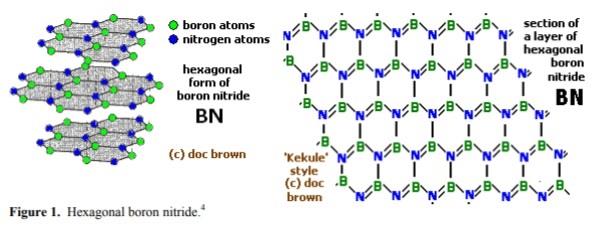
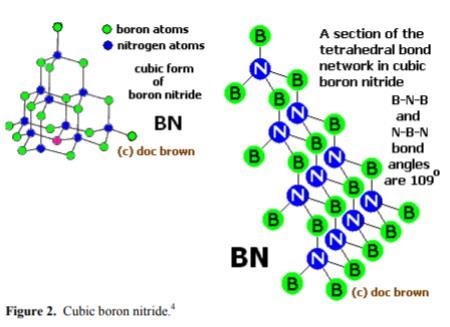
Boron nitride can also be in cubic form in which alternately linked boron and nitrogen atoms form a tetrahedral bond network, similar to carbon atoms in diamond.
Chemische Eigenschaften
white powder(s), 1μm or less 99.5% pure; hexagonal, most common form: a=0.2504 nm, c=0.6661nm; fcc: a=0.3615nm; hardness: hexagonal like graphite,?cub approaches that of diamond; band gap ~7.5 eV at 300K; dielectric 7.1; used in furnace insulation and in crucibles for melting aluminum, boron, iron, and silicon, also as sputtering target for dielectrics, diffusion masks, passivation layers [KIR81] [HAW93] [MER06] [CER91]
Physikalische Eigenschaften
Insulator (
Eg=7.5 eV). Crucibles
for melting molten metals such
as Na, B, Fe, Ni, Al, Si, Cu, Mg,
Zn, In, Bi, Rb, Cd, Ge, and Sn.
Corroded by molten metals U,
Pt, V, Ce, Be, Mo, Mn, Cr, V, and
Al. Attacked by molten salts
PbO
2, Sb
2O
3, AsO
3, Bi
2O
3, KOH,
and K
2CO
3. Used in furnace
insulation-diffusion masks and
passivation layers.
Verwenden
Boron nitride is a material in which the extra electron of nitrogen (with respect to carbon) enables it to form structures that are isoelectronic with carbon allotropes. Also used in manufacture of alloys; in semiconductors, nuclear reactors, lubricants.
Hexagonal boron nitride can be used as an electrical insulator; as thermocouple protection sheaths, crucibles and linings for reaction vessels; and as a coating for refractory molds used in glass forming and in superplastic forming of titanium. It can also be incorporated in ceramics, alloys, resins, plastics, and rubber to give them self-lubricating properties. Hexagonal boron nitride is used the formulation of coatings and paints for high temperature applications. It is also used as a substrate for semi-conductors, lens coatings, and transparent windows.
https://www.cir-safety.org
Vorbereitung Methode
In tonnage production, acetaldehyde may be manufactured by:
1. The direct oxidation of ethylene, requiring a catalytic solution of copper chloride plus small quantities of palladium chloride Cl2Pd.
2. The oxidation of ethyl alcohol C2H6O with sodium dichromate Cr2Na2O7, and
3. The dry distillation of calcium acetate C4H6CaO4 with calcium formate C2H2CaO4.
Application
Boron nitride finds applications in shaping tools in industries due to its ability to withstand temperatures greater than 2,000°C. Cutting tools and abrasive components, designed specifically for use with low-carbon ferrous metals, have been developed using cubic boron nitride. These tools perform similarly to polycrystalline diamond (PCD) tools but can be utilized on iron and low-carbon alloys without the risk of a reaction occurring.
synthetische
Boron nitride is prepared by heating boric oxide with ammonia:
B
2O
3 + 2NH
3 → 2BN + 3H
2O
Alternatively, the compound can be prepared by heating boric oxide or boric acid with ammonium chloride or an alkali metal cyanide. Purified product can be obtained by high temperature reaction of boron halide with ammonia:
BCl
3 + NH
3 → BN + 3HCl
Boron nitride can also be made from the elements by heating boron and nitrogen at red heat.
Definition
boron nitride: A solid, BN, insolublein cold water and slowly decomposedby hot water; r.d. 2.25 (hexagonal);sublimes above 3000°C. Boronnitride is manufactured by heatingboron oxide to 800°C on an acid-solublecarrier, such as calcium phosphate,in the presence of nitrogen orammonia. It is isoelectronic with carbonand, like carbon, it has a veryhard cubic form (borazon) and asofter hexagonal form; unlikegraphite this is a nonconductor. It isused in the electrical industrieswhere its high thermal conductivityand high resistance are of especialvalue.
Allgemeine Beschreibung
Boron nitride in cubic form, known as Borazon, is a manufactured abrasive that was discovered by General Electric Co. Laboratories in 1957. Unlike manufactured diamond, Borazon does not have a natural counterpart. It is produced under temperatures and pressures similar to those required for diamond manufacture. Additionally, cubic boron nitride exhibits greater thermal stability compared to diamond. It remains stable at temperatures exceeding 1,371°C, while diamond reverts to graphite at temperatures above 816°C. Borazon has a Knoop hardness (K100) of 7800, which is higher than ordinary abrasives but lower than diamond.
Industrielle Verwendung
Boron nitride (BN) has many potential commercial applications. It is a white, fluffy powder with a greasy feel. It is used for heat-resistant parts by molding and pressing the powder without a binder to a specific gravity of 2.1 to 2.25.
BN may be prepared in a variety of ways, for example, by the reaction of boron oxide with ammonia, alkali cyanides, and ammonium chloride, or of boron halides and ammonia. The usually high chemical and thermal stability, combined with the high electrical resistance of BN, suggests numerous uses for this compound in the field of high-temperature technology. BN can be hot-pressed into molds and worked into desired shapes.
BN powders can be used as mold-release agents, high-temperature lubricants, and additives in oils, rubbers, and epoxies to improve thermal conductance of dielectric compounds. Powders also are used in metal- and ceramicmatrix composites (MMC and CMC) to improve thermal shock and to modify wetting characteristics.
The platy habit of the particles and the fact that boron nitride is not wet by glass favors use of the powder as a mold wash, e.g., in the fabrication of high-tension insulators. It is also useful as thermal insulation in induction heating. A cubic form of boron nitride (Borazon) similar to diamond in hardness and structure has been synthesized by the high-temperature, high-pressure process for making synthetic diamonds. Any uses it may find as a substitute for diamonds will depend on its greatly superior oxidation resistance.
Industrielle Verwendung
The major industrial applications of hexagonal boron nitride rely on its high thermal conductivity, excellent dielectric properties, self-lubrication, chemical inertness, nontoxicity, and
ease of machining. These are, for instance, mold wash for releasing molds, high-temperature
lubricants, insulating filler material in composite materials, as an additive in silicone oils and
synthetic resins, as filler for tubular heaters, and in neutron absorbers. On the other hand, the
industrial applications of cubic boron nitride rely on its high hardness and are mainly as
abrasives.
Forms and nomenclature
Boron nitride exists as three different poly-morphs:
Alpha-boron nitride (α-BN), a soft and ductile polymorph (ρ = 2280 kg.m
–3 and m.p. = 2700°C) with a hexagonal crystal lattice similar to that of graphite, also called hexagonal boron nitride
(HBN) or white graphite;
Beta-boron nitride (β-BN), the hardest manmade material and densest polymorph (ρ = 3480 kg.m
–3, m.p. = 3027°C), with a cubic crystal lattice similar to that of
diamond, also called cubic boron nitride (CBN) or borazon;
Pyrolitic boron nitride
(PBN). From a chemical point of view, boron nitride oxidizes readily in air at temperatures
above 1100°C, forming a thing protective layer of boric acid (H
3BO
3) on its surface that prevents further oxidation as long as it coats the material. Boron nitride is stable in reducing
atmospheres up to 1500°C.
Bornitrid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

