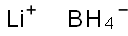Lithiumtetrahydroborat
|
|
Lithiumtetrahydroborat Eigenschaften
- Schmelzpunkt:
- 280 °C
- Siedepunkt:
- 66°C/760mmHg
- Dichte
- 0.896 g/mL at 25 °C
- Flammpunkt:
- −1 °F
- storage temp.
- water-free area
- L?slichkeit
- Soluble in ether, THF, and aliphatic aminesSoluble in ether, tetrahydrofuran, aliphatic amines and ethanol.
- Aggregatzustand
- Powder
- Farbe
- White
- Wichte
- 0.66
- Explosionsgrenze
- 4.00-75.60%(V)
- Wasserl?slichkeit
- soluble H2O above pH 7, ether, tetrahydrofuran, aliphatic amines [MER06]
- Sensitive
- Air & Moisture Sensitive
- Merck
- 14,5525
- CAS Datenbank
- 16949-15-8(CAS DataBase Reference)
- NIST chemische Informationen
- Lithium tetrahydroborate(16949-15-8)
- EPA chemische Informationen
- Borate(1-), tetrahydro-, lithium (16949-15-8)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | F,T,C,Xn,F+ | ||
|---|---|---|---|
| R-S?tze: | 14/15-23/24/25-34-20/21/22-11-40-36/37/38-19-67-66-22-12 | ||
| S-S?tze: | 26-36/37/39-43-45-36/37-16 | ||
| RIDADR | UN 3399 4.3/PG 1 | ||
| WGK Germany | 2 | ||
| RTECS-Nr. | ED2725000 | ||
| F | 10-21 | ||
| TSCA | Yes | ||
| HS Code | 2850 00 20 | ||
| HazardClass | 4.3 | ||
| PackingGroup | I |
| Bildanzeige (GHS) |
  
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | ||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||||||||||||||||
| Sicherheit |
|
Lithiumtetrahydroborat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R14/15:Reagiert heftig mit Wasser unter Bildung hochentzündlicher Gase.R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut.
R34:Verursacht Ver?tzungen.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R11:Leichtentzündlich.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S43:Zum L?schen . . . (vom Hersteller anzugeben) verwenden (wenn Wasser die Gefahr erh?ht, anfügen: "Kein Wasser verwenden").
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Chemische Eigenschaften
WHITE POWDERPhysikalische Eigenschaften
White orthorhombic crystals; density 0.67 g/cm3; decomposes in moist air; melts at 268°C; decomposes at 380°C; reacts with water; dissolves in ether, tetrahydrofuran, and diethylamine; solubility in ether, 25g/L at 25°C.Verwenden
Strong reducing agent. Used to reduce compounds containing ketones, aldehydes, ester carbonyls, acid chlorides, lactones, and epoxidesLithium borohydride is a versatile reducing agent for aldehydes, ketones, nitriles, primary amides, acid chlorides, lactones, epoxides, and esters. It is involved in the preparation of intercalation compounds and nanocomposites. It is used as a precursor for other borohydrides and acts as a catalyst in hydroboration reactions. It is known to be good reversible storage compound for anhydrous ammonia and hydrogen.Allgemeine Beschreibung
A white to grayish crystalline powder.Air & Water Reaktionen
Likely to ignite when moistened with water [Lab. Gov. Chemist 1965].Reaktivit?t anzeigen
Lithium borohydride is a strong reducing agent. Is easily ignited and burns vigorously once ignited. Reacts on contact with water or acids to form hydrogen gas and corrosive products. Reaction with limited amounts of water or moisture may cause ignition after a delay [Gaylord, 1965, p. 22].Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.Brandgefahr
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.Sicherheitsprofil
Poison by ingestion, inhalation, and skin contact. Flammable; can liberate H2. Incompatible with H20 as moisture on fibers of cellulose or as liquid. See also LITHIUM, BORON COMPOUNDS, and HYDRIDES.l?uterung methode
It is crystallised from Et2O, and pumped free of ether at 90-100o during 2hours [Schaeffer et al. J Am Chem Soc 78 729 1956]. Store it dry as it decomposes slowly in moist air. [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 775 1963.]Lithiumtetrahydroborat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
BENZYL 4-(BROMOMETHYL)TETRAHYDRO-1(2H)-PYRIDINECARBOXYLATE
3,5-Dimethoxybenzylamine
Methyl 3-formylbenzoate
Thiophen-2-ethylamin
(1-Aminocyclopropyl)methanol
1H-Azepine-3-carboxylicacid,hexahydro-,methylester(9CI)
2,4-DIMETHOXYPHENETHYLAMINE
(S)-4-BOC-MORPHOLINE-3-CARBOXYLIC ACID
5-(AMINOMETHYL)-1-METHYLPYRROLIDIN-2-ONE
16949-15-8(Lithiumtetrahydroborat)Verwandte Suche:
Sodium triacetoxyborohydride
Dilithiumoxid
Natriumcyantrihydroborat
Kaliumtetrahydroborat
Lithiumtetrafluoroborat, wasserfrei
Lithiumchlorid
Natriumtetrahydroborat
Lithiumhydrid
Dilithiumoxalat
Lithiumtetrahydroborat
Lithium
Bor
Lithium triisobutylhydroborate
Tetrahydrofuran
Lithiumtriethylhydroborat
Lithium-[2H4]tetrahydroborat(1-)
Lithiumtriethyl[2H]hydroborat(1-)
- Lithium boron tetrahydride
- Lithium borohydride, 4M (10 wt.%) solution in THF, AcroSeal
- LithiuM borohydride, 95% 10GR
- LithiuM borohydride, 95% 50GR
- LithiuM borohydride, 2.0 M solution in THF
- LithiuM borohydride solution 3M in THF
- LithiuM borohydrid
- LithiuM borohydride >=90%
- LithiuM borohydride >=95.0%
- LithiuM borohydride solution 2.0 M in THF
- borohydruredelithium
- Lithiumboranat
- Lithiumborhydrid
- Lithiumtetrahydridoborat
- Lithiumborohydride,95%
- Lithiumtetrahydroborat
- Borate,tetrahydro-,lithium
- Lithium Borohydride (ca. 3mol/L in Tetrahydrofuran)
- LITHIUM TETRAHYDRIDOBORATE
- Borate(1-), tetrahydro-, lithium
- Borate(1-),tetrahydro-,lithium
- LITHIUM BOROHYDRIDE
- LITHIUM BORON HYDRIDE
- LITHIUM BORANATE
- tetrahydro-borate(1-lithium
- LITHIUM BOROHYDRIDE, HYDROGEN STORAGE G&
- LITHIUM BOROHYDRIDE, 2.0M SOLUTION IN TE TRAHYDROFURAN
- Lityium borohydride
- Lithium borohydride, 2M solution in tetrahydrofuran
- LithiuM borohydride, 2.0 M solution in THF, SpcSeal
- LithiuM borohydride, 4.0 M solution in THF, SpcSeal
- Lithium borohydride, 2.0 M solution in THF, HySeal
- Lithium tetrahydroborate for synthesis
- Lithium Borohydride (ca. 4mol/L in Tetrahydrofuran)
- lithium:boron(1-)
- Lithium borohydride, 2.0 M solution in THF, J&KSeal
- ithium borohydride
- Lithium borohydride tetrahydrofuran solution
- Lithium Borohydride Powder
- Lithium borohydride2M solution in tetrahydrofuranAcroSeal§3
- Lithium borohydride (LBH)
- Lithium Borohydride in THF
- LiBH4
- lithium borohydride solution
- LITHIUM TETRAHYDROBORATE
- PQHL
- Lithiumborohydride 2. M in THF
- Lithium Borohydride-Tetrahydrofuran
- 16949-15-8
- 16949-66-2
- BH4Li
- H4BLi
- Classes of Metal Compounds
- Li (Lithium) Compounds
- Typical Metal Compounds
- Tetrahydroborates
- Reduction
- B (Classes of Boron Compounds)