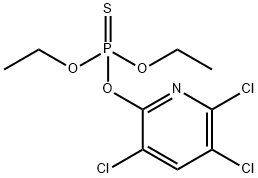
Chlorpyrifos (ISO)
|
|
Chlorpyrifos (ISO) Eigenschaften
- Schmelzpunkt:
- 42-44°C
- Siedepunkt:
- 200°C
- Dichte
- 1.398
- Dampfdruck
- 5.03 x 10-5 mmHg at 25 °C (subcooled liquid vapor pressure calculated from GC retention time data,Hinckley et al., 1990)
- Flammpunkt:
- 2 °C
- storage temp.
- APPROX 4°C
- L?slichkeit
- (At 25 °): 6.5, 7.9, 6.3, and 0.45 kg/kg in acetone, benzene, chloroform, and methanol, respectively (Worthing and Hance, 1991)
- Aggregatzustand
- solid
- pka
- -5.28±0.10(Predicted)
- Farbe
- White to off-white
- Wasserl?slichkeit
- Insoluble. 0.00013 g/100 mL
- Merck
- 13,2208
- BRN
- 1545756
- Henry's Law Constant
- 8.19 at 5 °C, 20.7 at 15 °C, 22.7 at 20 °C, 35.5 at 25 °C, 146 at 35 °C:in 3% NaCl solution: 32.3 at 5 °C, 82.9 at 15 °C, 301 at 25 °C, 535 at 35 °C (gas stripping-GC, Cetin et al., 2006)
- Expositionsgrenzwerte
- OSHA PEL: TWA 0.2 mg/m3, STEL 0.6 mg/m3; ACGIH TLV: TWA 0.2 mg/m3, STEL 0.6 mg/m3
- Stabilit?t:
- Stable. Incompatible with strong oxidizing agents.
- InChIKey
- SBPBAQFWLVIOKP-UHFFFAOYSA-N
- LogP
- 5.21 at 20℃
- CAS Datenbank
- 2921-88-2(CAS DataBase Reference)
- NIST chemische Informationen
- o,o-Diethyl-o-(3,5,6-trichloro-2-pyridyl)phosphorothioate(2921-88-2)
- EPA chemische Informationen
- Chlorpyrifos (2921-88-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | T;N,N,T,Xn,F,Xi | ||
|---|---|---|---|
| R-S?tze: | 25-50/53-36-20/21/22-11-38 | ||
| S-S?tze: | 1/2-45-60-61-36/37-26-16 | ||
| RIDADR | UN 2783 | ||
| OEB | C | ||
| OEL | TWA: 0.2 mg/m3, STEL: 0.6 mg/m3 [skin] | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | TF6300000 | ||
| HazardClass | 6.1(b) | ||
| PackingGroup | III | ||
| HS Code | 29333990 | ||
| Giftige Stoffe Daten | 2921-88-2(Hazardous Substances Data) | ||
| Toxizit?t | LD50 orally in rats: 145 mg/kg (Schafer) |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Chlorpyrifos (ISO) Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS WEISSE KRISTALLE MIT CHARAKTERISTISCHEM GERUCH.CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen auf etwa 160°C unter Bildung giftiger und ?tzender Rauche mit Chlorwasserstoff, Phosgen, Phosphoroxiden, Stickstoffoxidenund Schwefeloxiden. Reagiert mit starken Basenund S?uren.ARBEITSPLATZGRENZWERTE
TLV: (Einatembarer Dampf und Aerosol) 0.1 mg/m?(als TWA); Hautresorption; Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); BEI vorhanden; (ACGIH 2005).MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation des Aerosols, über die Haut und durch Verschlucken.INHALATIONSGEFAHREN
Eine gesundheitssch?dliche Partikelkonzentration in der Luft kann beim Versprühen oder Dispergieren schnell erreicht werden, vor allem als Pulver.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:M?glich sind Auswirkungen auf das Nervensystem mit nachfolgenden Kr?mpfen und Atemdepression. Cholinesterasehemmer. Exposition weit oberhalb der Arbeitsplatzgrenzwerte kann zum Tod führen. Die Auswirkungen treten u.U. verz?gert ein. ?rztliche Beobachtung notwendig.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Cholinesterasehemmer. Kumulative Wirkung m?glich (s. AKUTE GEFAHREN/SYMPTOME).LECKAGE
Chemikalienschutzanzug mit umgebungsluftunabh?ngigem Atemschutzger?t. NICHT in die Umwelt gelangen lassen. Verschüttetes Material in Beh?ltern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste sorgf?ltig sammeln. An sicheren Ort bringen.R-S?tze Betriebsanweisung:
R25:Giftig beim Verschlucken.R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R36:Reizt die Augen.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R11:Leichtentzündlich.
R38:Reizt die Haut.
S-S?tze Betriebsanweisung:
S1/2:Unter Verschluss und für Kinder unzug?nglich aufbewahren.S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Beschreibung
Chlorpyrifos is a chlorinated organophosphorus (OP) ester manufactured as an insecticide, acaricide, and miticide. Like the other OP insecticides, the most prominent toxicity of chlorpyrifos is associated with binding and inhibition of the enzyme acetylcholinesterase (AChE) in insects and mammals. Chlorpyrifos requires metabolic activation to chlorpyrifos oxon to yield anticholinesterase activity.First sold in 1965, chlorpyrifos is used globally to control agricultural and structural pests and mosquitos. In the 1990s, chlorpyrifos ranked as one of the top selling pesticides in the world, for the most part, replacing the persistent organochlorine insecticides. Over the last decade, concerns regarding toxicity to the developing nervous system have limited its use. By 2001, residential uses and uses in schools and parks were prohibited, and many agricultural uses were restricted and the US Residential use limitations were also imposed in Canada, Australia, and the European Union (EU). It continues to be used in large quantities to control crop damage worldwide. In the developing countries, excessive agricultural application and lack of protective devices result in hundreds of thousands of deaths yearly.
Chemische Eigenschaften
Chlorpyrifos belongs to the class of insecticides known as organophosphates. Technical chlorpyrifos is an amber to white crystalline solid with a mild sulfur odor. It is insoluble in water, but soluble in benzene, acetone, chloroform, carbon disulfi de, diethyl ether, xylene, methylene chloride, and methanol. Formulations of chlorpyrifos include emulsifi able concentrate, dust, granular wettable powder, microcapsule, pellet, and sprays. Chlorpyrifos is widely used as an active ingredient in many commercial insecticides, such as Dursban and Lorsban, to control household pests, mosquitoes, and pests. Formulations of chlorpyrifos include emulsifi able concentrates, granules, wettable powders, dust, microcapsules, pellets, and sprays. The US EPA has classifi ed chlorpyrifos as a GUPPhysikalische Eigenschaften
Chlorpyrifos is a white crystalline or irregularly flaked solid. Chlorpyrifos has a very faint mercaptan-type odor. Chlorpyrifos is not soluble in water. Chlorpyrifos can cause slight irritation to the eye and skin.Verwenden
Chlorpyrifos belongs to a class of insecticides known as organophosphates. Technical chlorpyrifos is amber to white crystalline solid with a mild sulphur odour. Formulations of chlorpyrifos include emulsifiable concentrate, dust, granular wettable powder, microcapsule, pellet, and sprays. Chlorpyrifos is widely used as an active ingredient in many commercial insecticides such as Dursban and Lorsban to control household pests, mosquitoes, and pests in animal houses. The U.S. EPA classified chlorpyrifos as GUP.Definition
ChEBI: An organic thiophosphate that is O,O-diethyl hydrogen phosphorothioate in which the hydrogen of the hydroxy group has been replaced by a 3,5,6-trichloropyridin-2-yl group.Air & Water Reaktionen
Insoluble in water. Chlorpyrifos reacts with water and most reactive hydrogen compounds. The rate of hydrolysis in water increases with pH, with temperature and with the presence of copper and possibly other metals that can form chelates.Reaktivit?t anzeigen
Chlorpyrifos is sensitive to heat and is decomposed by moisture. Chlorpyrifos is hydrolyzed by strong alkalis. Chlorpyrifos is corrosive to copper and brass. Chlorpyrifos is also corrosive to copper alloys. Chlorpyrifos reacts with water and most reactive hydrogen compounds. The rate of hydrolysis in water increases with pH, with temperature and with the presence of copper and possibly other metals that can form chelates.Health Hazard
Exposures to chlorpyrifos cause adverse health effects and poisoning. The symptoms include, but are not limited to, headache, dizziness, respiratory problems, muscular and joint pains, numbness, tingling sensations, incoordination, tremor, nausea, abdominal cramps, vomiting, sweating, blurred vision, respiratory depression, slow heart beat, nervousness, weakness, cramps, diarrhea, chest pain, pin-point pupils, tearing, salivation, clear nasal discharge and sputum, muscle twitching, and in severe poisonings convulsions, coma, and death. Exposures to chlorpyrifos cause adverse effects to the nervous system. The effects include phosphorylation of the active site, disturbance in the activity of the acetylcholinesterase (AChE) enzyme (inactivity). AChE enzyme is necessary to stop the transmission of the chemical neurotransmitter. In occupational workers, high concentrations of chlorpyrifos cause poisoning with symptoms of unconsciousness, convulsions and/or fatal injury. Persons with respiratory ailments and disturbed liver function are known to be at increased health risk. Also, repeated exposures to chlorpyrifos have been reported to cause disturbances in the process of brain development.Brandgefahr
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Landwirtschaftliche Anwendung
Insecticide, Nematicide: A U.S. EPA restricted Use Pesticide (RUP). Chlorpyrifos is one of the most widely used insecticides in the U.S., both around the home and in agriculture. A broad-spectrum insecticide, originally used primarily to kill mosquitoes but no longer registered for that use. Chlorpyrifos is effective in controlling cutworms, corn rootworms, cockroaches, grubs, flea beetles, flies, termites, fire ants, and lice. It is used as an insecticide on grain, cotton, field, fruit, nut and vegetable crops, as well as on lawns and ornamental plants. It is also registered for direct use on sheep and turkeys, for horse site treatment, dog kennels, domestic dwellings, farm buildings, storage bins, and commercial establishments. Chlorpyrifos acts on pests primarily as a contact poison, with some action as a stomach poison. It is available as granules, wettable powder, dustable powder and emulsifiable concentrate. Top crop uses in California include cotton, alfalfa, almonds, and oranges.Handelsname
(Note: EPA Office of Pesticide Programs lists 2135 products, both active and past-registered) ALUDOR®; BAR 500 EC®; BRODAN®; CHLORBAN®; CHLORPIRIFOS 480 CE MILENIA®; CHOIR®; COROBAN®; CURIGNA®; CYREN®; DETMOL U. A. ®; DORSAN®; DORSAN®-C; DOWCO® 179; DURSBAN®; EF 121®; EMPIRE®; ERADEX®; GLOBAL CRAWLING INSECT BAIT®; KENSBAN®; LORSBAN®; MURPHY SUPER ROOT GUARD®; PAQEANT®; PILOT®; PYRINEX®); SCOUT®; SPANNIT®; STIPEND; TALON®; TAFABAN®; TERIAL®; TWINSPAN®Sicherheitsprofil
Poison by ingestion, intraperitoneal, skin contact, and inhalation routes. Human systemic effects by ingestion: paresthesia, muscle weakness, coma. Experimental reproductive effects: developmental toxicity. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cl-, NOx POx and SOxm?gliche Exposition
A potential danger to those involved in the manufacture, formulation, and application of this insecticide.Carcinogenicity
Some recent studies have reported associations between chlorpyrifos exposure and increased risk for cancer for farm workers participating in the Agricultural Health Study. Specifically, increased risk for glioma and rectal cancer has been associated with chlorpyrifos exposure . Chlorpyrifos was also one pesticide associated with trends toward higher incidence of lung cancer through 2001 . For all cancers though, follow- up periods are short and exposures are based on recall so results may be unreliable.Environmental Fate
Biological. From the first-order biotic and abiotic rate constants of chlorpyrifos in estuarine water and sediment/water systems, the estimated biodegradation half-lives were 3.5–41 and 11.9–51.4 days, respectively (Walker et al., 1988)Soil. Hydrolyzes in soil to 3,5,6-trichloro-2-pyridinol (Somasundaram et al., 1991). The half-lives in a silt loam and clay loam were 12 and 4 weeks, respectively (Getzin, 1981). In another study, Getzin (1981a) reported the hydrolysis half-lives
Leoni et al. (1981) reported that the major degradation product of chlorpyrifos in soil is 3,5,6-trichloro-2-pyridinol. The major factors affecting the rate of degradation include chemical hydrolysis in moist soils, clay-catalyzed hydrolysis on dry soil s
Plant. The half-life of chlorpyrifos in Bermuda grasses was 2.9 days (Leuck et al., 1975). The concentration and the formulation of application of chlorpyrifos will determine the rate of evaporation from leaf surfaces. Reported foliar half-lives on tomato, orange and cotton leaves were 15–139, 1.4–96 and 5.5–57 hours, respectively (Veierov et al., 1988). Dislodgable residues of chlorpyrifos on cotton leaf 0, 24, 48, 72 and 96 hours after application (1.1 kg/ha) were 3.64, 0.13, 0.071, 0.055 and 0.034 μg/m2, respectively (Buck et al., 1980)
Surface Water. In an estuary, the half-life of chlorpyrifos was 24 days (Schimmel et al., 1983).
Stoffwechselwegen
The metaboic fate of chlorpyrifos in soil, plants and animals is similar, with oxidative dealkylation or hydrolysis to diethyl phosphorothioate and 3,5,6-trichloro-2-pyridinol being the major route of detoxification. The latter metabolite is conjugated as the glycosides or glucuronides in plants and animals. De-ethylation is not a major route of detoxification in mammals. Activation by desulfuration to the active acetylcholinesterase inhibitor, chlorpyrifos oxon, occurs in both animals and plants but the compound is often not detected owing to its rapid rate of hydrolysis. Dechlorination of the chloropyridine ring also occurs in the environment, principally by photolysis.Versand/Shipping
UN2783 Organo phosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Inkompatibilit?ten
Above 130°C this chemical may undergo violent exothermic decomposition. The substance decomposes on heating at approximately 160°C and on burning, producing toxic and corrosive fumes including hydrogen chloride, nitrogen oxides; phosphorous oxides, sulfur oxides. Reacts with strong acids; strong bases; causing hydrolysis. Attacks copper and brass. Contact with oxidizers may cause the release of phosphorous oxides. Contact with strong reducing agents, such as hydrides, may cause the formation of flammable and toxic phosphine gas.Waste disposal
This compound is 50% hydrolyzed in aqueous MeOH solution at pH 6 in 1930 days; and in 7.2 days at pH 9.96. Spray mixtures of <1% concentration are destroyed with an excess of 5.25% sodium hypochlorite in <30 minutes @ 100°C; and in 24 hours @ 30°C. Concentrated (61.5%) mixtures are essentially destroyed by treatment with 100:1 volumes of the above sodium hypochlorite solution and steam in 10 minutes. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Vorsichtsma?nahmen
Occupational workers should be careful during handling and use of chlorpyrifos. The workplace should have adequate washing facilities at all times and close to the site of handling and use. Eating, drinking, and smoking should be prohibited during handling and before washing after handling. Containers should be kept away from foodstuffs, animal feed and their containers, and out of reach of children.Chlorpyrifos (ISO) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1-Methylimidazol
Natrium-3,5,6-trichlorpyridin-2-olat
Benzyltriethylammoniumchlorid
Natriumchlorid
2,3,5,6-Tetrachloropyridine
Chlor
Pentachlorpyridin
2,3,6-Trichloropyridine
3,5,6-Trichlor-2-pyridon
Trichloracetylchlorid
4-Hydroxy-3-methylacetophenon
Dichlormethan
Chlorthiophosphors?ure-0,0-diethylester
Chinalphos (ISO)
Trichloressigsure
Downstream Produkte
2921-88-2(Chlorpyrifos (ISO))Verwandte Suche:
Ethanol
Ethylacetat
Triethylphosphat
Ethyl-4-hydroxybenzoat
Fenitrothion (ISO)
Fenthion (ISO)
Ethylacrylat
Ethyl cellulose
Chlorethan
Tris(2-chlorethyl)-phosphat
Diethylcarbonat
Mefenpyr-diethyl
Diethylmalonat
Phoxim (ISO)
Tris(trimethylsilyl)phosphat
Ethylbenzol
Diethylphthalat
Diazinon (ISO)
- dursban hf
- DURSBAN(R)
- DOWCO 179
- Eradex
- CHLORPYRIFOS
- Chlorpyritos
- CYREN(R)
- CUGAT
- ENT 27311
- ent27,311
- ent27311
- ENT-27311
- epapesticidecode059101
- Equity
- Ethion, dry
- ethion,dry
- Killmaster
- Lentrek
- Lock-On
- lorsban50sl
- Loxiran
- m-Chlorpyrifos
- O,O-Diaethyl-O-3,5,6-trichlor-2-pyridylmonothiophosphat
- Super I.Q.A.P.T.
- Phosphorothioic acid O,O-diethyl O-(3,5,6-trichloro-2-pyridinyl) ester
- PYRINEX(R)
- O,O-DIETHYL-O-[3,5,6-TRICHLORO-2-PYRIDYL]-PHOSPHOROTHIOATE
- O,O-DIETHYL O-(3,5,6-TRICHLORO-2-PYRIDYL)-THIOPHOSPHATE
- NUFOS(R)
- LORSBAN
- lorsban 4e-sg
- TRICHLORPYRPHOS
- CHLORPYRIFOS 94
- Affront
- chloropyrifos solution
- chlorpyrifos (bsi,e-iso,ansi,esa,ban)
- chlorpyrifos solution
- CHLORPYRIFOS 97%TC
- DIETHYL-3,5,6-TRICHLORO-2-PYRIDYLPHOSPHOROTHIOATE
- ETHYL-1-CHLORPYRIFOS
- O,O-DIETHYL-O-(3,5,6-TRICHLORO-2-PYRIDYL)PHOPHOROTHIOATE
- Ohlorpyrifos
- DURSBAN, 100MG, NEAT
- CHLORPYRIFOS, 1GM, NEAT
- CHLORPYRIPHOS PESTANAL, 250 MG
- CHLOROPYRIFOS, 1X1ML, MTBE, 1000UG/ML
- CHLORPIRYIFOS
- 0,0-Diethyl 0-(3,5,6-trichloropyridyl ester thiophophoric acid
- chlorpyrifos (ISO) O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate
- Chlorpyrifos, >=97%
- DIMETHYL SULFOXIDE (DMSO) ACS GRADE
- FERRIC AMMONIUM SULFATE 12H2O ACS GRADE
- Chlorpyrifos, Pesticide Mix
- Phosphorothioic acid O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
- Chlorpyrifos Standard
- diethoxy-sulfanylidene-(3,5,6-trichloropyridin-2-yl)oxy-$l^{5}-phosphane
- diethoxy-thioxo-[(3,5,6-trichloro-2-pyridyl)oxy]phosphorane
- Chlorpyrifos, Chlorpyriphos