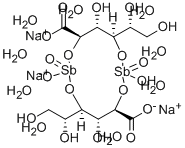
Sodium Stibogluconate
|
|
Sodium Stibogluconate Eigenschaften
- Schmelzpunkt:
- >250°C (dec,)
- storage temp.
- 2-8°C
- L?slichkeit
- H2O: 1 mg/mL at ~75 °C
- Aggregatzustand
- solid
- Farbe
- pale yellow
- Wasserl?slichkeit
- water: 1mg/mL
- Stabilit?t:
- Hygroscopic
- InChIKey
- VJCZMGLJTJZFEN-ZYPIZMQFSA-K
- CAS Datenbank
- 16037-91-5
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn,N | ||
|---|---|---|---|
| R-S?tze: | 20/22-51/53 | ||
| S-S?tze: | 61 | ||
| RIDADR | 3282 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | CC7930000 | ||
| HazardClass | 6.1(b) | ||
| PackingGroup | III | ||
| Toxizit?t | LD50 ipr-mus: 33 mg/kg CLDND* 11,155,49 |
| Bildanzeige (GHS) |
 
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Sodium Stibogluconate Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Sodium stibogluconate is a pentavalent antimony derivative that is highly active against Leishmania amastigotes in macrophages. Sodium stibogluconate has diverse effects on both this intracellular parasite and its host cell.Verwenden
Sodium stibogluconate is a pentavalent antimony derivative that is highly active against Leishmania amastigotes in macrophages. Sodium stibogluconate has diverse effects on both this intracellular parasite and its host cell.Antimicrobial activity
It has low activity against the extracellular promastigote stage of Leishmania spp. in vitro, but is active against amastigotes in macrophages. The trivalent form is considered to be the toxophore, with metabolism of SbV to toxic SbIII in the host cell macrophage and the parasite; the level of metabolism is higher in amastigotes than promastigotes. Variation in the sensitivity of different Leishmania species may contribute to differences in clinical response. It is more active against visceral than cutaneous leishmaniasis in animal models. Sodium stibogluconate cures CNS infections with T. brucei in rodents.Acquired resistance
Unresponsiveness and relapse of L. donovani infections in Bihar State, India (over 60% of cases), is due to increasing acquired resistance. In laboratory-generated and clinical isolates, resistant Leishmania promastigotes show increased levels of intracellular thiols, for example trypanothione, to which SbIII is conjugated and extruded by efflux pumps.Lack of response is also reported in patients with mucosal leishmaniasis caused by L. braziliensis. Relapse is common in patients with visceral leishmaniasis who are immunosuppressed, for example by HIV infection, but this is due to pathogen interaction and the immune dependence of drug activity and not acquired resistance. High mortality has been reported in treatment of these co-infection cases.
Pharmazeutische Anwendungen
A pentavalent antimonial of uncertain chemical composition; probably a complex mixture of polymeric forms. There is batchto- batch variation and solutions may contain 32–34% pentavalent antimony (SbV). The structural formula is conjectural as studies have identified a mixture of non-complexed SbV and large polymeric gluconate complexes. Chemical composition also depends on concentration and time. It is freely soluble in water.Pharmakokinetik
Peak concentrations of about 12–15 mg antimony/L are achieved in serum 1 h after a dose of 10 mg/kg. There is a slow accumulation in the central compartment, and tissue concentrations reach a maximum after several days. In contrast to trivalent derivatives, pentavalent antimonials are not accumulated by erythrocytes, but there is evidence of protein binding. Antimony is detected in the skin for at least 5 days after treatment. Some of the dose of SbV is converted to SbIII, possibly by the liver or by macrophages. It is rapidly excreted into urine with a half-life of about 2 h; 60–80% of the dose appears in the urine within 6 h of parenteral administration. In a study on structurally related meglumine antimonate the pharmacokinetics of SbV and SbIII were similar as measured by serum and urine levels.Clinical Use
Sodium antimony gluconate (Pentostam) is a pentavalent antimonialcompound intended primarily for the treatment ofvarious forms of leishmaniasis. It is available from the CDCas the disodium salt, which is chemically stable and freelysoluble in water. The 10% aqueous solution used for eitherintramuscular or intravenous injection has a pH of approximately5.5. Like all antimonial drugs, this drug has a lowtherapeutic index, and patients undergoing therapy with itshould be monitored carefully for signs of heavy metal poisoning.Other organic antimonial compounds are used primarilyfor the treatment of schistosomiasis and other flukes.The antileishmanial action of sodium stibogluconate requiresits reduction to the trivalent form, which is believedto inhibit phosphofructokinase in the parasite.Nebenwirkungen
The toxic effects are limited by the rapid excretion, but cumulative toxicity increases in proportion to dose. Myalgia, arthralgia, anorexia and electrocardiographic changes have been reported with high-dose regimens. In particular, development of ventricular tachyarrhythmias associated with prolongation of the QT interval has been recorded. Hepatocellular damage, hepatic and renal functional impairment and pancreatitis have also been reported. The changes are reversible on discontinuation of treatment.Sicherheitsprofil
Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of Sb.Sodium Stibogluconate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Sodium Stibogluconate Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 122)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Eaglech(shanghai)Pharmaceutical Co., Ltd | +86-2160290089 +86-13884467586 |
eagpharm@gmail.com | China | 97 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 |
sales@sdperfect.com | China | 294 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19552 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32202 | 58 |
| Longyan Tianhua Biological Technology Co., Ltd | 0086 18039857276 18039857276 |
CHINA | 2783 | 58 | |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
16037-91-5()Verwandte Suche:
Lithiumcarbonat
Lobelin
Aluminiumhydroxid
Testosteronpropionat
Vitamin-A-s?ure
Ethanol
Kohlenstoffdioxid
Harnstoff
Azathioprin
Suxamethoniumchlorid
Antimonnatriumtartrat
Mercaptopurin
Natriumgluconat
D-Allose
- SODIUM STIBOGLUCONATE
- SSG
- NASB
- NASBV
- d-gluconicacid,2,4:2’,4’-o-(oxydistibylidyne)bis-,sb,sb’-dioxide,trisodium
- d-gluconicacid,cyclicesterwithantimonicacid(h8sb2o9)(2:1),trisodium
- estibogluconatosodico
- myostibin
- pentostam
- salt,nonahydrate
- solustibosan
- solustin
- solusurmin
- solyusurmin
- stibanate
- stibanose
- stibatin
- stibinol
- ANTIMONY SODIUM GLUCONATE
- Antimony(V) sodium gluconate
- Sodium stibagluconate
- Aids007935
- Aids-007935
- Stibogluconate (sodium)
- Sodium Stibogluconate - CAS 16037-91-5 - Calbiochem
- Stibogluconate trisodium nonahydrate
- Sodium Stibogluconate USP/EP/BP
- D-Gluconic acid,2,4:2',4'-O-(oxydistibylidyne)bis-, sodium salt, hydrate (1:3:9)
- Phosphatase,inhibit,Sodium stibogluconate,Inhibitor
- 16037-91-5
- C12H18Na3O17Sb2
- C12H37Na3O27Sb2
- C12H18Na2O17Sb2
- C12H17O17Sb23Na
- C12H17O17Sb29H2O3Na
- Cell Signaling and Neuroscience
- Cell Biology
- BioChemical
- Kinase/Phosphatase Biology
- Tyrosine Phosphatase (TP)
- Tyrosine Phosphatase Inhibitors