123040-16-4
中文名稱
鹽酸阿扎司瓊
英文名稱
Azasetron hydrochloride
CAS
123040-16-4
分子式
C17H21Cl2N3O3
MDL 編號
MFCD00209913
分子量
386.27
MOL 文件
123040-16-4.mol
更新日期
2025/05/26 09:19:27
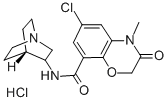 123040-16-4 結(jié)構(gòu)式
123040-16-4 結(jié)構(gòu)式
基本信息
中文別名
鹽酸阿扎司瓊N-(1-氮雜雙環(huán)[2.2.2]辛-8-基)-6-氯-4-甲基-3-氧代-3,4-二氫-2H-1,4-苯并惡嗪-8-甲酰胺鹽酸鹽
鹽酸阿扎司瓊
英文別名
AZASETRONAZASETRON HCL
AZASETRON HYDROCHLORIDE
N-1-AZABICYCLO[2.2.2]-OCT-3-YL-6-CHLORO-3,4-DIHYDRO-4-METHYL-3-OXO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE, HYDROCHLORIDE
N-(1-AZABICYCLO[2.2.2]OCT-3-YL)-6-CHLORO-4-METHYL-3-OXO-3,4-DIHYDRO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE HCL
N-(1-AZABICYCLO[2.2.2]OCT-3-YL)-6-CHLORO-4-METHYL-3-OXO-3,4-DIHYDRO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE HYDROCHLORIDE
Y-25130
Y-25130 HYDROCHLORIDE
2h-1,4-benzoxazine-8-carboxamide,3,4-dihydro-n-1-azabicyclo(2.2.2)oct-3-yl-6-c
N-(1-Azabicyclo[2,2,2]Oct-3-Yl)-6-Chloro-4-Methyl-3-Oxo-3,4-Dihydro-2h-1,4-Benzoxazine-8-Carboxamide HCl
N-(1-AZABICYCLO[2.2.2]OCT-3-YL)-6-CHLORO-4-METHYL-3-OXO-3,4-DIHYDRO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE HYDROCHLORIDE
Y-25130HCl
N-(1-Azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzo
INTERMEDIATE OF AZASETRON HCL
Serotone
Azasetron hydrochloride
N-(1-Azabicyclo[2.2.2]octan-8-yl)-6-chloro-4-methyl-3-oxo-1,4-benzoxazine-8-carboxamide hydrochloride
N-(1-Azabicyclo[2.2.2]octane-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide·hydrochloride
所屬類別
原料藥:止吐催吐藥物理化學(xué)性質(zhì)
外觀白色粉末。
熔點281° (dec); mp 305° (dec) (Kawakita)
儲存條件Sealed in dry,Room Temperature
溶解度甲醇(微溶)、水(微溶)
溶解度少許溶于甲醇和水
形態(tài)白色固體。
顏色白色
水溶解性H2O: 2mg/mL, clear
CAS 數(shù)據(jù)庫123040-16-4(CAS DataBase Reference)
貯存方式-20℃低溫保存.
原研廠家Japan Tobacco (Originator), Mitsubishi Pharma (Originator), Taiho (Not Determined), Torii (Marketer), Temis-Lostalo (Licensee).
應(yīng)用領(lǐng)域
用途一
鹽酸阿扎司瓊【123040-16-4】是抗生素類抗感染原料藥,用于細胞毒類藥物引起的惡心、嘔吐。 用于放療引起的惡心、嘔吐。用于婦科手術(shù)及外科手術(shù)引起的惡心、嘔吐。鹽酸阿扎司瓊價格(試劑級)
| 報價日期 | 產(chǎn)品編號 | 產(chǎn)品名稱 | CAS號 | 包裝 | 價格 |
| 2025/05/22 | 46507 | 鹽酸阿扎司瓊 Azasetron hydrochloride | 123040-16-4 | 250mg | 2550元 |
| 2025/05/22 | 46507 | 鹽酸阿扎司瓊 Azasetron hydrochloride | 123040-16-4 | 1g | 7071元 |
| 2025/05/22 | HY-B0068 | 鹽酸阿扎司瓊 Azasetron hydrochloride | 123040-16-4 | 5 mg | 375元 |
常見問題列表
生物活性
Azasetron HCl (Y-25130) 是一種選擇性5-HT3受體拮抗劑,IC50為0.33 nM,用于癌癥化療引起的惡心和嘔吐。靶點
| Target | Value |
| 5-HT3 | 0.33 nM |
體內(nèi)研究
Azasetron could effectively penetrate through the skin and pass into the systemic circulation.
| Animal Model: | Four male Bama miniature pigs weighing 9-11 kg (15-16 weeks old). |
| Dosage: | 0.5 mg/kg. |
| Administration: | I.V. administration via the abdominal vein. |
| Result: | The mean plasma concentration dropped to minimum at 36 h. C max = 44.88 ± 7.16 ng/mL) was achieved at the time point of 66.00 ± 22.98 h (T max ). |

