| Identification | More | [Name]
1-Phenyltetrazole-5-thiol | [CAS]
86-93-1 | [Synonyms]
1H-TETRAZOLE, 1-PHENYL-5-THIOL
1-PHENYL-1H-1,2,3,4-TETRAAZOLE-5-THIOL
1-PHENYL-1H-TETRAZOLE-5-THIOL
1-PHENYL-2-TETRAZOLINE-5-THIONE
1-PHENYL-5-MERCAPT-1H-TETRAZOLE
1-PHENYL-5-MERCAPTO-1,2,3,4-TETRAZOLE
1-PHENYL-5-MERCAPTO-1H-TETRAZOLE
1-PHENYL-5-MERCAPTOTETRAZOLE
1-Phenyltetrazole-5-thiol
1-PHENYLTETRAZOLINE-5-THIONE
5-MERCAPTO-1-PHENYL-1,2,3,4-TETRAZOLE
5-MERCAPTO-1-PHENYL-1H-TETRAZOLE
5-MERCAPTO-1-PHENYLTETRAZOLE
PMT
TIMTEC-BB SBB007594
1,2-dihydro-1-phenyl-5h-tetrazole-5-thion
1,2-dihydro-1-phenyl-5H-Tetrazole-5-thione
1-phenyl-1h-tetrazole-5-thio
1-phenyl-1tetrazole-5-thiol
1-Phenyl-5-tetrazolethione | [EINECS(EC#)]
201-710-5 | [Molecular Formula]
C7H6N4S | [MDL Number]
MFCD00003129 | [Molecular Weight]
178.21 | [MOL File]
86-93-1.mol |
| Chemical Properties | Back Directory | [Appearance]
white to slightly yellow crystals or fluffy powder | [Melting point ]
145 °C (dec.)(lit.)
| [Boiling point ]
342°C (rough estimate) | [density ]
1.3046 (rough estimate) | [refractive index ]
1.5500 (estimate) | [Fp ]
138°C | [storage temp. ]
Store at +15°C to +25°C. | [solubility ]
ethanol: soluble5%, clear, colorless to faintly yellow | [form ]
Solid | [pka]
-3.85±0.20(Predicted) | [color ]
White to Off-White | [PH]
3-5 (H2O, 20℃)suspension | [Water Solubility ]
Soluble in 5% ethanol, water (partly), acetone, chloroform, and methanol. | [Sensitive ]
Air Sensitive | [Detection Methods]
T,NMR | [BRN ]
139068 | [InChIKey]
GGZHVNZHFYCSEV-UHFFFAOYSA-N | [CAS DataBase Reference]
86-93-1(CAS DataBase Reference) | [NIST Chemistry Reference]
1H-tetrazole-5-thiol, 1-phenyl-(86-93-1) | [EPA Substance Registry System]
86-93-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,F | [Risk Statements ]
R5:Heating may cause an explosion.
R22:Harmful if swallowed. | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 1325 4.1/PG 2
| [WGK Germany ]
1
| [RTECS ]
XF7700000
| [F ]
4.10 | [Hazard Note ]
Flammable | [TSCA ]
Yes | [HazardClass ]
4.1 | [PackingGroup ]
III | [HS Code ]
29339990 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Sodium azide-->Phenyl isothiocyanate-->S,S-bis(1-phenyl-1H-tetrazol-5-yl) dithiocarbonate-->Chlorzoxazone-->1H-Tetrazole, 5-bromo-1-phenyl--->5-ANILINO-1,2,3,4-THIATRIAZOLE-->N-METHYL-2-DIMETHYLAMINOACETOHYDROXAMIC ACID-->4-PHENYL-3-THIOSEMICARBAZIDE-->5-CHLORO-1-PHENYL-1H-TETRAZOLE-->2-Amino-4-chlorophenol-->Aniline | [Preparation Products]
Cefoperazone-->Cefazolin-->Cefazolin sodium salt-->2-Benzoxazolinone-->2,3-Dihydropyrido[2,3-d][1,3]oxazol-2-one-->2-Hydroxybenzimidazole-->2-Benzothiazolol-->1,4-Benzenediol, 2-[(1-phenyl-1H-tetrazol-5-yl)thio]- |
| Hazard Information | Back Directory | [Chemical Properties]
white fluffy or crystalline powder or crystals | [Uses]
1-Phenyl-1H-tetrazole-5-thiol is used as a corrosion inhibitor and as a reagent in the synthesis of Microcarpalide, a microfilament disrupting agent that is weakly cytotoxic to mammalian cells. | [Application]
1-Phenyl-1H-tetrazole-5-thiol is an effective inhibitor of aluminum corrosion in 1M HCl solution. It was used in the synthesis of oxacyclic building blocks via highly stereoselective radical cyclization and olefin metathesis reactions. It was also used in the synthesis of metallated tetradecyl sulfone. It is also used in the spectrophotometric determination of Pt and Bi.
| [Definition]
ChEBI:1-Phenyl-5-mercaptotetrazole is a member of tetrazoles. | [Synthesis Reference(s)]
Canadian Journal of Chemistry, 44, p. 2315, 1966 DOI: 10.1139/v66-347 | [Synthesis]
Sodium azide, 0.79 g (12.1 mmol), and 1.65 g (12.1 mmol) of ZnCl2 were added to 30 mL of MeCN. The mixture was heated with stirring until boiling (80 °C), and then 1 g (10.1 mmol) of allyl isothiocyanate was added. The reaction mixture was stirred for 1 h at 80 °C, and the solvent was then removed in a vacuum. The residue was treated with 5% aqueous NaOH (50 mL) with stirring for 20 min. The suspension was filtered, and the filtrate was treated with chloroform (2×10 mL) to remove impurities. The aqueous layer was acidified with conc. HCl to pH 1. The formed precipitate was filtered off, washed with water, and dried in air to afford 1-Phenyltetrazole-5-thiol. Yield 1.31 g(99%), colorless crystals.
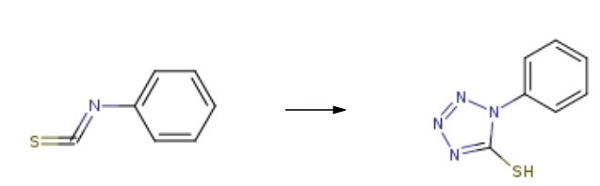
| [Purification Methods]
Purify the tetrazole by recrystallisation from EtOH or CHCl3 (m 152o) [Tautomerism: Kauer & Sheppard J Org Chem 32 3580 1967, UV: Leiber et al. Can J Chem 37 563 1959]. The ammonium salt crystallises from EtOH and decomposes at 176o. The sodium salt crystallises from EtOH/*C6H6, melts at 96o and decomposes at 145o [Stollé J Prakt Chem [2] 133 60 1932]. It is used for the determination of Bi and Pd. [Fresenius Z Anal Chem 261 151 1972, Beilstein 26 III/IV 2065.] |
|
|