| Identification | More | [Name]
Aztreonam | [CAS]
78110-38-0 | [Synonyms]
2-[(2-AMINO-THIAZOL-4-YL)-(2-METHYL-4-OXO-1-SULFO-AZETIDIN-3-YLCARBAMOYL)-METHYLENEAMINOOXY]-2-METHYL-PROPIONIC ACID
[2S-[2A,3B(Z)]]-2-[[[1-(2-AMINO-4-THIAZOLYL)-2-[(2-METHYL-4-OXO-1-SULFO-3-AZETIDINYL)AMINO]-2-OXOETHYLIDENE]AMINO]OXY]-2-METHYLPROPANOIC ACID
(2S-(2ALPHA,3BETA(Z)))-2-(((1-(2-AMINO-4-THIAZOLYL)-2-((2-METHYL-4-OXO-1-SULFO-3-AZETIDINYL)AMINO)-2-OXOETHYLIDENE)AMINO)OXY)-2-METHYL-PROPANOIC ACID
2-[[[(Z)-1-(2-AMINO-4-THIAZOLYL)-2-[[(2S,3S)-2-METHYL-4-OXO-1-SULFO-3-AZETIDINYL]AMINO]-2-OXOETHYLIDENE]AMINO]OXY]-2-METHYLPROPANOIC ACID
AZACTAM
AZTHREONAM
AZTREONAM
(2s-(2-alpha,3-beta(z)))-dinyl)amino)-2-oxoethylidene)amino)oxy)-2-methyl
antibioticsquibb26,776
monobactam
propanoicacid,2-(((1-(2-amino-4-thiazolyl)-2-((2-methyl-4-oxo-1-sulfo-3-azeti
sq26,776
AZTREONAM, E-ISOMER USP STANDARD
AZTREONAM, USP STANDARD
Azteronam
[2S-[2a,3b(Z)]]-2-[[(Z)-[1-(2-Amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic Acid
Aztreon
Nebactam
Squibb 2677
[2s-[2α,3β(z)]]-2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic acid | [EINECS(EC#)]
278-839-9 | [Molecular Formula]
C13H17N5O8S2 | [MDL Number]
MFCD00072145 | [Molecular Weight]
435.43 | [MOL File]
78110-38-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
227°C | [density ]
1.83 | [refractive index ]
1.6460 (estimate) | [storage temp. ]
Store at 0-5°C | [solubility ]
DMSO (Slightly), Water (Slightly, Heated, Sonicated) | [form ]
solid
| [pka]
pKa -0.7(H2O t=RT Iunde?ned) (Uncertain);2.75(H3O t=RT Iunde?ned) (Uncertain);3.91(H4O t=RT Iunde?ned) (Uncertain) | [color ]
white to beige | [Water Solubility ]
Soluble in DMF/water (1:1) at 50 mg/ml | [Usage]
The first totally synthetic monocyclic ?lactam antibiotic | [Merck ]
14,925 | [InChIKey]
WZPBZJONDBGPKJ-VEHQQRBSSA-N | [SMILES]
C(O)(=O)C(O/N=C(/C1=CSC(N)=N1)\C(N[C@@H]1C(=O)N(S(O)(=O)=O)[C@H]1C)=O)(C)C | [CAS DataBase Reference]
78110-38-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
2
| [RTECS ]
UA2451400
| [HS Code ]
2941906000 | [Toxicity]
TDLo ivn-rat: 1100 mg/kg (7-17D preg):TER NKRZAZ33(Suppl 1),203,85 |
| Hazard Information | Back Directory | [Description]
Aztreonam is the first member of the monobactam class of antibiotics to be
introduced into the world market. It possesses high β-lactamase stability and
moderately good activity against gram negative aerobes such as E. coli, S. marcescens,
-9 Proteus Providencia, Salmonella, g. influenzae, E. gonorrhea, and &. pneumonia.
While somewhat less potent against Pseudomonas aeruginosa, it is nonetheless one
of the better β-lactams against this species. It has poor activity against gram
positive organisms. | [Originator]
Squibb (USA) | [History]
Aztreonam was synthesized by the Squibb Institute for Medical Research in 1981 starting with l-threonine. The synthesis was based on findings about bacterial β-lactam compounds of a monocyclic nature . The β-lactam compounds, called monobactams, were isolated from Chromobacterium violaceum, Agrobacterium radiobacter, etc. Such monocyclic βlactams of bacterial origin had previously been found independently in 1981 by Takeda Chemicals Industries in the culture broths of Pseudomonas acidophila and P. mesoacidophila and named sulfazecin and isosulfazecin, respectively. Aztreonam was selected from among hundreds of derivatives as a candidate for clinical trials because of its unique antibacterial spectrum and strong activity. This antibiotic shows excellent activity against a variety of gram-negative aerobic bacteria but no activity against gram-positive bacteria or anaerobes. Its efficacy and safety are now being clinically evaluated. | [Manufacturing Process]
This mixture was sterilized for 15 minutes at 121°C at 15 lbs/inch2 steam
pressure prior to use. The fermentation flasks were incubated at 25°C for 40
to 45 hours on a of rotary shaker. A 250 liter batch of Agrobacterium
radiobacter A.T.C.C. No. 31700 is fermented in a 100 gallon steel vessel with
a media and operating conditions described below. Culture of Agrobacterium
radiobacter grown out on agar slants, pH 7.3 consisted of yeast extract (1 g),
beef extract (1 g), NZ amine A (2 g), glucose (10 g), agar (15 g) in 1000 ml
distilled water. Loopful of surface growth from agar slant was used as the
source of incolumn. Medium of oatmeal (20 g), tomato paste (20 g) tapped
water to 1000 ml, pH 7, was sterilized for 15 min at 121°C at 15 lbs/inch2
steam pressure prior to use. 100 ml of the medium, containing incolumn is
incubated at 25°C for about 24 hours on a rotary shaker. It was added to a
mixture of yeast extract (5 g), glucose (10 g) in 1 L distilled water and
incubated for about 42 hours at 25°C in 100 gallon stainless steel
fermentation vessel.
During incubation, the broth is agitated at 155 r.p.m. and aerated at rate of
10.0 cubic feet per minute. An antifoam agent (Ucon LB625, Union Carbide)
was added as needed. The fermentation beer was adjusted to pH 4 with
aqueous HCl and calls separated by centrifugation. The supernatante (200 L) was extracted with 40 L of 0.05 m cetyldimethylbenzyl ammonium chloride in
dichloromethane and extract concentrated in vacuo to 5.5 L. The concentrate
was then extracted with solution of 177 g of sodium thiocyanate in 2 L of
water, adjusting the mixture of pH 4.35 with phosphoric acid. The aqueous
extract was concentrated in vacuo to 465 ml and added to 1840 ml of
methanol. Solids are filtrated yielded 194 g of crude solid product. It was
dissolved and chromatographed on a 5x106.5 cm column of Sephadex G-10
three times and after concentrating in vacuo gave 3.5 g of crude antibiotic
M53 (azetreonam) which was chromatographed at first on QAE Sephadex A-
25 (liner gradient, prepared from 2.5 L of water and 2.5 L of 0.25 M sodium
nitrate). Then the residue (fractions 26-75) gave M53 (natrium salt) after
evaporation. It was triturated with methanol and the souble fraction, 0.40 g
was chromatographed on a 2.5x20 cm column of Diaion HP20AG, eluting at 2
ml per minute with water and collecting 20 ml fractions. Fractions 26-75 gave
51.9 mg of antibiotic M53 (sodium salt). | [Brand name]
Azactam (Bristol-Myers Squibb);PRIMBACTAM. | [Therapeutic Function]
Antibiotic | [Antimicrobial activity]
Concentrations (mg/L) inhibiting
50% of other organisms are: Aeromonas spp., 0.1;Acinetobacter spp., 16; Mor. catarrhalis, 0.1; Burkholderia cepacia,
2; and Yersinia spp., 0.1. Synergy has been shown with gentamicin,
tobramycin and amikacin against 52–89% of strains of Ps.
aeruginosa and gentamicin-resistant Gram-negative bacteria. | [General Description]
Azactam (aztreonam for injection, intravenous or intramascular)contains the active ingredient aztreonam, which is amember of the monobactam class of antibiotics. A true antibiotic,aztreonam was originally isolated from cultures ofthe bacterium Chromobacterium violaceum. Now, the antibioticis prepared by total synthesis. Monobactams possessa unique monocyclic β-lactam nucleus, and are structurallyunlike other β-lactams like the penicillins, cephalosporins,carbapenems, and cephamycins. The β-lactam arrangementof aztreonam is unique, possessing an N-sulfonic acid functionality.This group activates the β-lactam ring towardattack. The side chain (3-position) aminothiazolyl oximemoiety and the 4-methyl group specify the antibacterialspectrum and β-lactamase resistance.
The mechanism of action of aztreonam is essentially identicalto that of other β-lactam antibiotics. The action of aztreonamis inhibition of cell wall biosynthesis resulting from ahigh affinity of the antibiotic for penicillin binding protein 3(PBP-3). Unlike other β-lactam antibiotics, aztreonam doesnot induce bacterial synthesis of β-lactamases. The structureof aztreonam confers resistance to hydrolysis by penicillinasesand cephalosporinases synthesized by most Gramnegativeand Gram-positive pathogens. Because of theseproperties, aztreonam is typically active against Gram-negativeaerobic microorganisms that resist antibiotics hydrolyzedby -lactamases. Aztreonam is active against strains that aremultiply-resistant to antibiotics such as cephalosporins, penicillins,and aminoglycosides. The antibacterial activity ismaintained over a broad pH range (6–8) in vitro, as well as inthe presence of human serum and under anaerobic conditions.
Aztreonam for injection is indicated for the treatment ofinfections caused by susceptible Gram-negative microorganism,such as urinary tract infections (complicated and uncomplicated),including pyelonephritis and cystitis(initial and recurrent) caused by E. coli, K. pneumoniae, P.mirabilis, P. aeruginosa, E. cloacae, K. oxytoca, Citrobactersp., and S. marcescens. Aztreonam is also indicated for lowerrespiratory tract infections, including pneumonia and bronchitiscaused by E. coli, K. pneumoniae, P. aeruginosa, H.influenzae, P. mirabilis, S. marcescens, and Enterobacterspecies. Aztreonam is also indicated for septicemia causedby E. coli, K. pneumoniae, P. aeruginosa, P. mirabilis, S.marcescens, and Enterobacter spp. Other infections respondingto aztreonam include skin and skin structure infections,including those associated with postoperative wounds andulcers and burns. These may be caused by E. coli, P.mirabilis, S. marcescens, Enterobacter species, P. aeruginosa,K. pneumoniae, and Citrobacter species. Intra-abdominalinfections, including peritonitis caused by E. coli,Klebsiella species including K. pneumoniae, Enterobacterspecies including E. cloacae, P. aeruginosa, Citrobacterspecies including C. freundii, and Serratia species includingS. marcescens. Some gynecologic infections, including endometritisand pelvic cellulitis caused by E. coli, K. pneumoniae,Enterobacter species including E. cloacae, and P.mirabilis also respond to aztreonam. | [Biochem/physiol Actions]
Aztreonam is a monobactam antibiotic used primarily to treat gram-negative bacterial infections. It is an older compound being re-examined as a therapeutic agent because of increasing carbapenem resistance in aerobic Gram-negative bacilli and because aztreonam is stable to Ambler class B metallo-β-lactamases. It is used alone or more commonly in combination with β-lactamase inhibitors such as avibactim. | [Pharmacokinetics]
Cmax 1 g intravenous: 90 mg/L end infusion
1 g intramuscular: 46 mg/L after 1 h
Plasma half-life: 1.7 h
Volume of distribution: 0.18 L/kg
Plasma protein binding: 56%
Absorption and distribution
Oral bioavailability is less than 1%. Peak concentrations
above the median MIC for most Gram-negative pathogens
are achieved in most tissues and body fluids after 1 g intramuscular
or intravenous doses.
Metabolism and excretion
It is not extensively metabolized, the most prominent product,
resulting from opening the β-lactam ring, being scarcely
detectable in the serum and accounting for about 6% of the
dose in the urine and 3% in the feces.
It is predominantly eliminated in the urine, where 58–72%
appears within 8 h. Less than 12% is eliminated unchanged in
the feces, suggesting low biliary excretion. | [Clinical Use]
Urinary tract infections, including pyelonephritis and cystitis
Lower respiratory tract infections, including pneumonia and bronchitis
caused by Gram-negative bacilli
Septicemia
Skin and skin structure infections, including postoperative wounds, ulcers
and burns
Intra-abdominal infections, including peritonitis
Gynecological infections, including endometritis and pelvic cellulitis | [Side effects]
Local reactions occasionally occur at the injection site.
Systemic reactions include diarrhea, nausea and/or vomiting
and rash (1–1.3%). Neutropenia was seen in 11.3% of the
pediatric patients younger than 2 years. Pseudomembranous
colitis has been reported.
There are no reactions in patients with immunoglobulin E
(IgE) antibodies to benzylpenicillin or penicillin moieties. It
is rarely cross-reactive with other β-lactam antibiotics and is
weakly immunogenic. | [Safety Profile]
Moderately toxic by severalroutes. An experimental teratogen. Other experimentalreproductive effects. When heated to decomposition itemits toxic fumes of NOx and SOx. | [Synthesis]
Aztreonam, (Z)-2[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-aze�tidinyl]cabamoyl]methylen]amino]oxy]-2-methylpropionoic acid (32.1.4.9), is synthesized
from tert-butyloxycarbonylthreonine, which is reacted with O-benzylhydroxylamine in the pres�ence of dicyclohexylcarbodimide and 1-hydroxybenzotriazole, to form the benzyl hydroxamide
derivative (32.1.4.1). This product undergoes a reaction with triphenylphosphine and ethyl
azodicarboxylate, which results in the cyclodehydration of the product to (3S-trans)-N-benzy�loxy-3-tert-butyloxycarbonylamino-4-methyl-azetidinone (32.1.4.2). Debenzylating this by
hydrogen reduction using a palladium on carbon catalyst forms (3S-trans)-N-hydroxy-3-tert�butyloxycarbonyl-amino-4-methyl-azetidinone (32.1.4.3). The hydroxyl group in this com�pound is removed by reducing it with titanium trichloride, which forms azetidinone (32.1.4.4).
Removing the tert-butyloxycarbonyl protection using trifluoroacetic acid and subsequent acyla�tion of the resulting product with the benzyl chloroformate gives (3S-trans)-benzyloxycarbony�lamino-4-methylazetidinone (32.1.4.5). Sulfonating this product with a mixture of sulfur trioxide and dimethylformamide gives the corresponding N-sulfonic acid. Turning the resulting N�sulfonic acid into a potassium salt by reacting it with potassium hydrophosphate, followed by
replacing the potassium cation with a tetrabutylammonium cation by reacting it with tetrabuty�lammonium sulfate gives the product (32.1.4.6). Reducing this with hydrogen using a palladium
on carbon catalyst gives 3-amino-4-methyl-monobactamic acid (32.1.4.7). Acylating this with
(Z) 2-amino-|á-[[2-(diphenylmethoxy)-1,1-dimethyl-2-oxoethoxy]imino] 4-thiazoleacetic acid
in the presence of dicyclohexylcarbodiimide and 1-hydroxy-benzotriazole gives the diphenyl�methyl ester of the desired aztreonam (32.1.4.8), which is hydrolyzed to aztreonam (32.1.4.9)
using trifluoroacetic acid.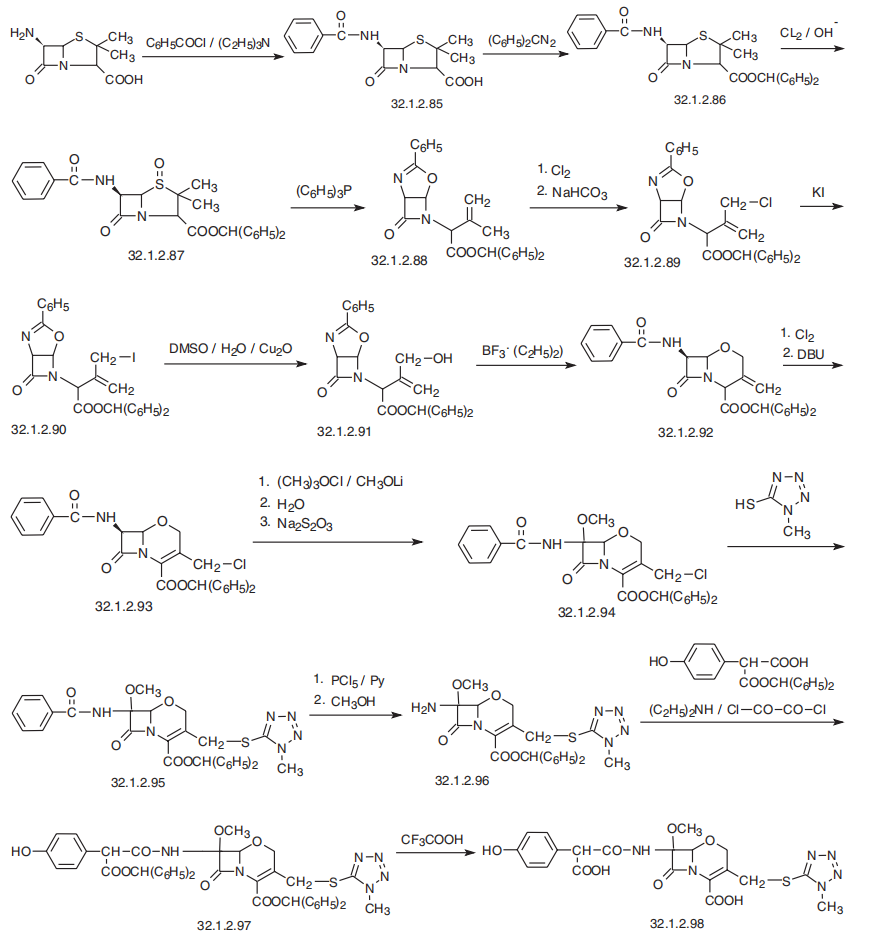
It is believed that the methyl group at position 4 increases the stability of the beta-lactam
ring with respect to most beta-lactamases, and at the same time it does not induce forma�tion of beta-lactamase as cephalosporins and imipenems do. | [Veterinary Drugs and Treatments]
Aztreonam is a monobactam antibiotic that may be considered for
use in small animals for treating serious infections caused by a wide
variety of aerobic and facultative gram-negative bacteria, including
strains of Citrobacter, Enterobacter, E. coli, Klebsiella, Proteus,
Pseudomonas and Serratia. The drug exhibits good penetration into
most tissues and low toxic potential and may be of benefit in treating
infections when an aminoglycoside or a fluoroquinolone are either
ineffective or are relatively contraindicated. Any consideration for
using aztreonam must be tempered with the knowledge that little clinical experience or research findings have been published with
regard to target species.
Aztreonam has also been used to treat pet fish (koi) infected
with Aeromonas salmonocida. | [Drug interactions]
Potentially hazardous interactions with other drugs
Possibly enhanced anticoagulant effect of coumarins. | [Metabolism]
Aztreonam is not extensively metabolised. The principal
metabolite, SQ-26992, is inactive and is formed by
opening of the beta-lactam ring; it has a much longer
half-life than the parent compound.
Aztreonam is excreted as unchanged drug with only small
quantities of metabolites, mainly in the urine, by renal
tubular secretion and glomerular filtration. Only small
amounts of unchanged drug and metabolites are excreted
in the faeces. | [storage]
Store at 0-6°C |
| Questions And Answer | Back Directory | [Outline]
Aztreonam is a novel kind ofβ-lactam antibiotics belonging to monobactams. In 1978 it was first discovered from the culture brot of New Jersey soil purple bacterium Chromobacterium violaceum, it has been obtained with synthesis method.
Mechanism
Aztreonam belongs to bacteria fungicides. It can quickly go through the outer membrane of Gram-negative aerobic bacteria wall, while it has a high affinity with the penicillin-binding protein 3 (PBP-3) . By acting on the PBP-3 and inhibiting synthesis of bacterial cell wall , it leads to cell lysis and death.
Aztreonam has a narrow antimicrobial spectrum, it only has antibacterial effect on aerobic gram-negative bacilli , such as E. coli, Klebsiella, Serratia spp, Proteus mirabilis, indole-positive Proteus, Citrobacter, influenza addicted blood coli, Pseudomonas aeruginosa and other Pseudomonas, some Enterobacter, Neisseria gonorrhoeae. The product is highly stable to β-lactamase produced by many bacteria .it is inactive in Gram-positive bacteria and anaerobic bacteria . Aztreonam compared with ceftazidime, gentamicin, it effect on aerogenes, and Enterobacter cloacae is higher than ceftazidime, but lower than gentamicin; the effect on Pseudomonas aeruginosa is lower than ceftazidime , and similar to gentamicin .
| [indications]
It is mainly used for the treatment of infections caused by susceptible strains which include the respiratory system, urinary, reproductive system infections (including acute gonorrhea), intra-abdominal infections, skin and soft tissue infections, before surgery preventing infections, other serious infections, such as sepsis.
| [Precautions ]
1.contraindication: patients allergic to the products or having a history of allergy on lidocaine and other local anesthetics or having anaphylactic shock on other β-lactam drugs are banned.
2. caution following:
It does not exist cross-allergic reactions between aztreonam and penicillins, but people having allergy body and allergic to penicillins, cephalosporins should take caution.
renal dysfunction patients should take caution.
aztreonam does not have toxicity for liver , but the patients having impaired liver function should observe the dynamic changes. It has been reported that in patients with alcoholic cirrhosis , the total clearance of the products can be reduced by 20-25%.
pregnant and lactating women, infants and young children should use with caution.
3. The impact of drugs on clinical outcomes:
direct anti-human globulin test (Coombs test) can be positive.
there may be a temporary increase of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and serum creatinine values after medication among a few patients, part thromboplastin time (PTT) and prothrombin time (PT) may be extended.
adverse reactions are skin symptoms such as skin rashes, purpura, pruritus (approximately 2%); gastrointestinal symptoms such as diarrhea, nausea, vomiting, taste changes, jaundice, and drug-induced hepatitis (approximately 2%); local irritation such as thrombophlebitis. Injection site swelling (approximately 2.4%); there are other neurological symptoms, vaginitis, oral lesions, fatigue, dizziness, and bleeding.
4. Long-term medication should pay attention to monitoring of liver, kidney and hematopoietic system.
The above information is edited by the chemicalbook of Tian Ye.
| [Chemical properties]
white or colorless powder crystals, melting at 227 ℃ (decomposition). It is dissolved in dimethyl formamide, slightly soluble in methanol, very slightly soluble in ethanol, insoluble in toluene, chloroform or ethyl acetate.
Aztreonam disodium: C13 H15N5Na2O8 . Acute toxicity LD50 (mg/kg): 3300 intravenous injection in mice, 6600 in rats intraperitoneally. | [Uses]
It is Mainly used for the infections caused by sensitive gram-negative bacteria , including pneumonia, pleurisy, abdominal infections, biliary tract infections, bone and joint infections, skin and soft tissue inflammation, especially for urinary tract infections, but also for sepsis. Because this product has good resistance to enzyme performance, therefore, when a microorganism is not sensitive to penicillins, cephalosporins, aminoglycosides and other drugs ,the product should often be effective.
| [production method]
Threonine for raw materials,chloride resulting from the chlorination forms an amide through ammonolysis, protect α-amino with benzyl chloroformate,esterifyβ-hydroxy with methanesulfonyl chloride , the amide group is sulfonated with sulfuric acid tetrabutylammonium , then it is cyclized to generate azetidine derivative in the potassium bicarbonate, then after hydrogen and Deprotection, produce 3-amino-2-methyl-4-oxo-1-sulfo-N oxetane.
Afetr dehydration amidation of side chains Azetidine derivative and thiazole derivatives obtained in the above, hydrolyse to deprotect and to obtain aztreonam.
| [Category]
Toxic substances
| [Toxicity grading]
Middle toxic
| [Acute toxicity]
Intraperitoneal-rat LD50: 2549 mg/kg; intraperitoneal-Mouse LD50: 2897 mg/kg.
| [Flammability and hazard characteristics]
Combustible; combustion produces toxic fumes of nitrogen oxides and sulfur oxides.
| [Storage Characteristics]
Ventilated, low-temperature ,dry storeroom.
| [Extinguishing agent]
Dry powder, foam, sand, carbon dioxide, water spray.
|
|
|