| Identification | More | [Name]
sec-Butanol | [CAS]
78-92-2 | [Synonyms]
(+/-)-2-BUTANOL
2-BUTANOL
2-HYDROXYBUTANE
BUTAN-2-OL
BUTYLENE HYDRATE
ETHYLMETHYLCARBINOL
METHYLETHYLCARBINOL
S-BUTYL ALCOHOL
SEC-BUTANOL
(+/-)-SEC-BUTYL ALCOHOL
SEC-BUTYL ALCOHOL
SECONDARY BUTYL ALCOHOL
(R,S)-Butan-2-ol
(RS)-2-butanol
1-Methyl propanol
1-methylpropanol
1-Methylpropyl alcohol
1-methylpropylalcohol
2-butanol,DL-
Alcool butylique secondaire | [EINECS(EC#)]
201-158-5 | [Molecular Formula]
C4H10O | [MDL Number]
MFCD00004569 | [Molecular Weight]
74.12 | [MOL File]
78-92-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Colourless liquid | [Melting point ]
−115 °C(lit.)
| [Boiling point ]
98 °C(lit.)
| [density ]
0.808 g/mL at 25 °C(lit.)
| [vapor density ]
2.6 (vs air)
| [vapor pressure ]
12.5 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.397(lit.)
| [Fp ]
80 °F
| [storage temp. ]
Flammables area | [solubility ]
125g/l | [form ]
Liquid | [pka]
>14 (Schwarzenbach et al., 1993) | [color ]
Colorless | [Odor]
Strong, pleasant. | [PH]
7 (20°C) | [PH Range]
7 | [Relative polarity]
0.506 | [Stability:]
Stable. Flammable. Substances to be avoided include acids, acid chlorides, acid anhydrides, oxidizing agents and halogens. | [explosive limit]
1.4-9.8%(V) | [Odor Threshold]
0.22ppm | [Odor Type]
fruity | [Water Solubility ]
12.5 g/100 mL (20 ºc) | [Sensitive ]
Hygroscopic | [Merck ]
14,1541 | [BRN ]
1718765 | [Henry's Law Constant]
1.19 (static headspace-GC, Merk and Riederer, 1997) | [Dielectric constant]
15.8(25℃) | [Exposure limits]
TLV-TWA 450 mg/m3 (150 ppm) (NIOSH),
305 mg/m3 (100 ppm) (ACGIH); IDLH
10,000 ppm. | [InChIKey]
BTANRVKWQNVYAZ-UHFFFAOYSA-N | [LogP]
0.610 | [Uses]
Preparation of methyl ethyl ketone, sol-
vent, organic synthesis, paint removers, industrial
cleaners.
| [CAS DataBase Reference]
78-92-2(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Butanol(78-92-2) | [EPA Substance Registry System]
78-92-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R10:Flammable.
R36/37:Irritating to eyes and respiratory system .
R67:Vapors may cause drowsiness and dizziness. | [Safety Statements ]
S13:Keep away from food, drink and animal feeding stuffs .
S24/25:Avoid contact with skin and eyes .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S46:If swallowed, seek medical advice immediately and show this container or label .
S7/9:Keep container tightly closed and in a well-ventilated place . | [OEB]
A | [OEL]
TWA: 100 ppm (305 mg/m3), STEL: 150 ppm (455 mg/m3) | [RIDADR ]
UN 1120 3/PG 3
| [WGK Germany ]
1
| [RTECS ]
EO1750000
| [Autoignition Temperature]
761 °F | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29051490 | [Safety Profile]
Poison by intravenous
and intraperitoneal routes. Mildly toxic by
ingestion. Experimental reproductive
effects. A skin and eye irritant. See also nBUTYL ALCOHOL and ALCOHOLS.
Dangerous fire hazard when exposed to heat
or flame. Auto-oxidizes to an explosive
peroxide. Ignites on contact with chromium
trioxide. To fight fire, use water spray,
alcohol foam, CO2, dry chemical.
Incompatible with oxidizing materials.
When heated to decomposition it emits
acrid smoke and fumes. | [Hazardous Substances Data]
78-92-2(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 6480 mg/kg LD50 dermal Rat > 2000 mg/kg | [IDLA]
2,000 ppm |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid with an alcohol odor. Flash point below 0 °F. Less dense than water. Vapors heavier than air. Soluble in water. Moderately irritates the eyes and skin. Prolonged and repeated contact may cause defatting and drying of the skin. Vapors may irritate the nose, throat and respiratory tract. May be harmful by ingestion. | [Reactivity Profile]
Attacks plastics. [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water (Merck 11th ed. 1989). Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73 1967; J, Org. Chem. 28:1893 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969]. | [Air & Water Reactions]
Highly flammable. Soluble in water. | [Hazard]
Toxic, mutagenic, upper respiratory tract
irritant, central nervous system impairment.
| [Potential Exposure]
Butyl alcohols are used as solvents for
paints, lacquers, varnishes, natural and synthetic resins,
gums, vegetable oils, dyes, camphor, and alkaloids. They
are also used as an intermediate in the manufacture of pharmaceuticals and chemicals; in the manufacture of artificial
leather, safety glass; rubber and plastic cements, shellac,
raincoats, photographic films, perfumes; and in plastic
fabrication.
| [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing
overexposure, as pulmonary edema may be delayed. As
first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy | [Shipping]
UN1120 Butanols, Hazard Class: 3; Labels: 3—
Flammable liquid. UN1212 Isobutanol or Isobutyl alcohol,
Hazard Class: 3; Labels: 3—Flammable liquid | [Incompatibilities]
Butyl alcohols may form explosive mixture with air. In all cases they are Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks
some plastics, rubber and coatings. n-Butanol is incompatible with strong acids; halogens, caustics, alkali metals; aliphatic amines; isocyanates. sec-Butanol forms an explosive
peroxide in air. Ignites with chromium trioxide.
Incompatible with strong oxidizers; strong acids; aliphatic
amines; isocyanates, organic peroxides. tert-Butanol is
incompatible with strong acids (including mineral acid),
including mineral acids; strong oxidizers or caustics, aliphatic amines; isocyanates, alkali metals (i.e., lithium,
sodium, potassium, rubidium, cesium, francium). isoButanol is incompatible with strong acids; strong oxidizers;
caustics, aliphatic amines; isocyanates, alkali metals and
alkali earth. May react with aluminum at high temperatur | [Waste Disposal]
Incineration, or bury absorbed
waste in an approved land fill. | [Physical properties]
Clear, colorless, flammable liquid with a pleasant odor. Experimentally determined detection and
recognition odor threshold concentrations were 400 μg/m3 (120 ppbv) and 1.2 mg/m3 (410 ppbv),
respectively (Hellman and Small, 1974). | [Definition]
ChEBI: A secondary alcohol that is butane substituted by a hydroxy group at position 2. | [Production Methods]
2-Butanol is produced commercially by the indirect hydration
of n-butenes. | [Health Hazard]
Exposure to 2-butanol may cause irritationof the eyes and skin. The latter effect isproduced by its defatting action on skin. Thistoxic property is mild and similar to thatof other butanol isomers. High concentrationmay produce narcosis. The narcotic effect isstronger than that of n-butanol, probably dueto the higher vapor pressure of the secondaryalcohol.
The toxicity is lower than that of itsprimary alcohol analogue.
LD50 value, oral (rats): 6480 mg/kg. | [Flammability and Explosibility]
Flammable | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Environmental Fate]
Biological. Bridié et al. (1979) reported BOD and COD values of 2.15 and 2.49 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a period of 5 d. The ThOD for sec-butyl alcohol is
2.59 g/g. In activated sludge inoculum, following a 20-d adaptation period, 98.5% COD removal
was achieved. The average rate of biodegradation was 55.0 mg COD/g?h (Pitter, 1976).
Photolytic. The estimated half-life of sec-butyl alcohol for the reaction of OH radicals in air
ranges from 129 d to 23 yr (Anbar and Neta, 1967).
Chemical/Physical. sec-Butyl alcohol will not hydrolyze in water because it does not contain a
hydrolyzable group (Kollig, 1993). | [Purification Methods]
Purification methods are the same as for n-Butanol. These include drying with K2CO3 or CaSO4, followed by filtration and fractional distillation, refluxing with CaO, distillation, then refluxing with magnesium and redistillation, and refluxing with, then distilling from CaH2. Calcium carbide has also been used as a drying agent. The anhydrous alcohol is obtained by refluxing with sec-butyl phthalate or succinate. (For method see Ethanol.) Small amounts of alcohol can be purified via conversion to the alkyl hydrogen phthalate and recrystallisation [Hargreaves J Chem Soc 3679 1956]. For purification of optical isomers, see Timmermans and Martin [J Chem Phys 25 411 1928]. [Beilstein 2 III 1566.] | [Toxics Screening Level]
The screening level for Sec-Butanol is 3000 μg/m3 based on 8 hour averaging.
|
| Questions And Answer | Back Directory | [Physical and Chemical Properties]
2-butanol is also known as methyl ethyl alcohol, chemical formula is CH3CH2CHOHCH3. Molecular weight is 74.12. It is colorless liquid with a strong aroma of mint, flammable, volatile, with optical activity. The molecule has a chiral carbon atom, can be present in three forms of right-handed body, left-handed body and racemic body, dl-body: the relative density is 0.8063. Melting point is-114.7 ℃. Boiling point is 99.5 ℃, 45.5 ℃ (7.999 × 103Pa). The flash point is 34 ℃. The refractive index is 1.3978. d-body: the relative density is 0.8080. Boiling point is 99.5 ℃. The flash point is 24 ℃. The refractive index is 1.3954. Specific rotation is + 13.9 °. l-body: the relative density is 0.8070. Boiling point is 99.5 ℃. The flash point is 28 ℃. The refractive index is 1.3955. Specific rotation is-13.51 ° (25 ℃). 2-butanol oxidation can generate methyl ethyl ketone and acetic acid. Slightly soluble in water (25 ℃ when 12.5ml/100ml), dissolved in acetone, benzene, miscible with ethanol and ether. This product interacts with water to form an azeotropic mixture, the product content is 68%, the total boiling point is 88.5 ℃. Rat oral is LD506480mg/kg. 2-butanol is the main raw material for producing methyl ethyl ketone, butyl acetate, sec-butyl acetate, and also used as a solvent and extraction agent, the raw materials of plasticizers, processing agents, herbicides, but also for synthesis of spices, flavors, coloring agents, wetting agent, cleaning agents and solvents of many natural resin, linseed oil and castor oil. | [Chemical Properties]
It is slightly sticky colorless flammable liquid, with a strong odor. Melting point is-114.7 ℃, the boiling point is 99.5 ℃, relative density (d204) is 0.808~0.809, the refractive index (nD25) is 1.3949, a flash point is 23.9 ℃. It is soluble in water, miscible in ethanol and ether. | [Uses]
1. Used as extraction solvent, spices.
2. 2-butanol is Used as a solvent, and chromatography reagents
3. 2-butanol is Used for the production of intermediates of methyl ethyl ketone, for the preparation of butyl acetate, sec-butyl, used as plasticizers, processing agents, herbicides, solvents and so on.
4. Used for the production of intermediates of methyl ethyl ketone, for the preparation of butyl acetate, sec-butyl. | [Butanol isomers]
Butanol is an important industrial raw material, also known as hydroxy butane, a monohydric alcohol, the lowest level alcohol for the same family which can have two or more isomers, butanol has four kinds of isomers, namely n-butanol, 2-butanol, tert-butanol and isobutanol. Relative molecular mass is 74.12. Butanol has the common property of alcohols, such as water-like, reacts with metal to produce alkoxide, reacts with halogen acid to produce halogenated hydrocarbons, dehydrates into alkenes, oxidation (or dehydrogenation) reaction to produce aldehydes and acids, and reacts with organic acids or oxygen-containing inorganic acid to generate ester and so on. In the four isomers, the toxicity of n-butanol is minimal, the toxicity of the other three is not large, but their irritation is great, with irritation to the skin and mucous membrane. Inhalation of large vapors can cause coma. The maximum allowable concentration at workplace is 100 × 10-6. Butanol can be directly used as a solvent, extraction agent, dehydrating agent, plasticizer, mineral processing agent, anti-aging agent, herbicide and so on.
Physical and chemical properties and toxicity of butanol are different due to isomers. The solubility in water depends on their structure, chemical properties depend on hydroxy location in alcohol: n-butanol and iso-butanol are primary alcohols, can be oxidized into the corresponding aldehyde or acid, sec-butyl alcohol is oxidized into the corresponding ketone, t-butanol is unsusceptible to oxidation.
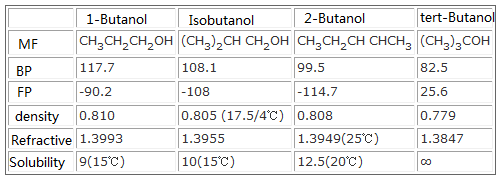
Physicochemical properties comparison chart of 4 isomers of butanol
Under dehydrating catalyst, butanol can produce butene, n-butanol and 2-butene can give 1-butene, 2-butene, 2-butanol can generate 2-butene, isobutanol and tert-butanol generate isobutylene. Under the catalyst of copper and silver, the dehydrogenation generates carbonyl compounds, n-butanol generates butyraldehyde, butanol generates methyl ethyl ketone, isobutanol generate isobutyraldehyde. Under the catalyst, air oxidation can generate acid. Catalyzed by a mineral acid, it reacts with organic acid to generate ester. Reaction with benzene, can generate butyl benzene. Butanol reacts with chlorine to generate butyraldehyde chloride. Under the action of the aluminum catalyst at 300~350 ℃, reacts with ammonia, n-butanol, iso-butanol and 2-butanol react with ammonia to generate butylamine, dibutylamine, tributylamine, t-butanol does not have this character. N-butanol and tert-butanol at 180 ℃ react with hydrogen sulfide to generate butyl mercaptan.
The above information were collated and edited by Xiaonan of Chemicalbook. | [Laboratory method for preparing 2-butanol]
1, As raw materials 2-butene reacts with sulfuric acid in concentrated sulfuric acid to produce sulfuric acid butyl ester, sulfuric acid ester is then hydrolyzed to produce 2-butanol, and then distillation purification.
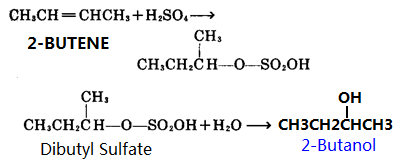
2, 2-butanone is used as raw material, under the action of a Grignard reagent, to prepare 2-butanol.
3, cis-2-butene is used as raw material, under the action of boron hydrides, to prepare d-or l-body. | [Production method]
After adsorption by butane of the cracking petroleum or natural gas in sulfuric acid, then hydrolyze with steam. | [Dangerous situations]
Prolonged inhalation is toxic, it irritates eye and skin. Flammable, flash point is 406 ℃, there is a greater risk of combustion. The allowable concentration in air of US is 100ppm (305mg/m3). | [Incompatibility]
Sec-butyl alcohol is incompatible with strong oxidizing agents. | [Storage]
It is Stored in metal drums, to prevent mechanical damage, best stored in a cool, dry and ventilated, non-flammable place, away from all possible sources of ignition, separated from strong oxidants. | [Transport requirements]
During transportation, "flammable liquid" shall be marked logo on the container. Others should be the same with "n-butanol". | [Extinguish measures]
When firing, dry powder fire extinguishing agent, fire-resistant foam or CO2 can be used. Water fighting is invalid, but spray with water in the fire container to keep it cool. If spills, and spills are not lighted, water mist can wash spray spills from the fire, and dilute to non-flammable mixtures. If necessary, water mist can be used to protect the operator to stop the leakage. Other items see the "n-butanol." | [Hazards & Safety Information]
Category: Flammable liquid
Toxicity grading: Poisoning
Acute toxicity
Oral-rat LD50: 6480 mg/kg, Intravenous-Mouse LD50: 764 mg/kg
Stimulus data
Eyes-rabbit 100 mg/24 hours moderate, Skin-rabbit 500 mg/24 hr mild
Hazardous characteristics
It is explosive when mixed with air, self-oxidized to form explosive peroxides.
Flammability hazard characteristics
in case of fire, high temperature, oxidant, it is flammable, burning to generate irritation smoke, spontaneous combustion in contact with chromium trioxide.
Storage characteristics
Treasury ventilation low-temperature drying, and it is stored from oxidants.
Extinguishing agent
Dry powder, water spray, carbon dioxide, foam
Professional standards
TWA 100 PPM (310 mg/m3) |
|
|