| Identification | More | [Name]
N-(1-Methylethyl)-4-((2-methylhydrazino)methyl)benzamide | [CAS]
671-16-9 | [Synonyms]
n-(1-methylethyl)-4-((2-methylhydrazino)methyl)benzamide
PROCARBAZINE
1-Methyl-2-(p-(isopropylcarbamoyl)benzyl)hydrazine
2-(p-Isopropylcarbamoylbenzyl)-1-methylhydrazine
4-((2-Methylhydrazino)methyl)-N-isopropylbenzamide
Benzamide, N-(1-methylethyl)-4-[(2-methylhydrazino)methyl]-
cb400-497
ibenzmethyzin
Ibenzmethyzine
MIH
MIH hydrochloride
n-(1-methylethyl)-4-((2-methylhydrazino)methyl)-benzamid
n-(1-methylethyl)-4-((2-methylhydrazino)methyl-benzamid
n-4-isopropylcarbamoylbenzyl-n’-methylhydrazine
Nathulane
Natulan
Natulanar
N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide
n-isopropyl-alpha-(2-methylhydrazino)-p-toluamid
N-Isopropyl-alpha-(2-methylhydrazino)-p-toluamide | [EINECS(EC#)]
211-582-2 | [Molecular Formula]
C12H19N3O | [MDL Number]
MFCD00866411 | [Molecular Weight]
221.3 | [MOL File]
671-16-9.mol |
| Hazard Information | Back Directory | [Potential Exposure]
Procarbazine is available in capsule
form. The primary use of this drug is as an antineoplastic
agent in the treatment of advanced Hodgkin’s disease, and
oat-cell carcinoma of the lung. The hydrochloride com-
pound is used in treatment. The FDA approved use of pro-
carbazine hydrochloride in 1969 and indicated that the drug
should be used as an adjunct to standard therapy. Possible
exposure occurs during manufacture of the drug and direct
exposure during its subsequent administration to patients.
Some of the metabolites of procarbazine hydrochloride are
both carcinostatic and carcinogenic. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respira-
tion with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with run-
ning water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical obser-
vation is recommended for 24?48 hours after breathing
overexposure, as pulmonary edema may be delayed.
As first aid for pulmonary edema, a doctor or authorized
paramedic may consider administering a drug or other
inhalation therapy. | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. | [Incompatibilities]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or
explosions. Keep away from alkaline materials, strong
bases, strong acids, oxoacids, epoxides. | [Chemical Properties]
Procarbazine is a white to pale yellow crystal-
line powder with a slight odor. | [Waste Disposal]
It is inappropriate and
possibly dangerous to the environment to dispose of
expired or waste drugs and pharmaceuticals by flushing
them down the toilet or discarding them to the trash.
Household quantities of expired or waste pharmaceuticals
may be mixed with wet cat litter or coffee grounds,
double-bagged in plastic, discard in trash. Larger quanti-
ties shall carefully take into consideration applicable
DEA, EPA, and FDA regulations. If possible return the
pharmaceutical to the manufacturer for proper disposal
being careful to properly label and securely package
the material. Alternatively, the waste pharmaceutical
shall be labeled, securely packaged and transported by a
state licensed medical waste contractor to dispose by
burial in a licensed hazardous or toxic waste landfill
or incinerator. | [Uses]
antibacterial | [Uses]
Procarbazine was initially synthesized as an MAO inhibitor. However, it was discovered
later on that it has ability to act as an alkylating agent and inhibit DNA, RNA, and protein
synthesis. Along with this, there is an opinion that procarbazine accumulates in cancerous
tissue and generates peroxide and hydroperoxide radicals in cells, which imitates the effect
of ionizing radiation. It is used for malignant tumors of lymphatic tissue, brain tumors,
lung tumors, and Hodgkin’s disease. A synonym of this drug is natulan. | [Definition]
ChEBI: A benzamide obtained by formal condensation of the carboxy group of 4-[(2-methylhydrazino)methyl]benzoic acid with the amino group of isopropylamine. An antineoplastic chemotherapy drug used for treatment of Hodgkin's lymphoma. Metabolism yields azo-procar
azine and hydrogen peroxide, which results in the breaking of DNA strands. | [Indications]
Procarbazine (Matulane) may autooxidize spontaneously,
and during this reaction hydrogen peroxide
and hydroxyl free radicals are generated. These highly
reactive products may degrade DNA and serve as one
mechanism of procarbazine-induced cytotoxicity. Cell
toxicity also may be the result of a transmethylation reaction
that can occur between the N-methyl group of
procarbazine and the N7 position of guanine. | [Indications]
Procarbazine exhibits an interesting interaction with
ethanol, resulting in headaches, diaphoresis, and facial
erythema; patients taking this drug should be forewarned
to abstain from alcohol. Procarbazine is also a
monoamine oxidase (MAO) inhibitor and may potentiate
the effects of drugs that are substrates for this enzyme. | [Brand name]
Matulane (Sigma-Tau). | [Mechanism of action]
Procarbazine is rapidly absorbed after oral administration
and has a plasma half-life of only 10 minutes.
The drug crosses the blood-brain barrier, reaching levels
in CSF equal to those obtained in plasma.
Metabolism is extensive and complex. Urinary excretion
accounts for 70% of the procarbazine and its
metabolites lost during the first 24 hours after drug administration. | [Clinical Use]
When originally tested as a single agent in advanced
Hodgkin’s disease, procarbazine produced tumor regression
responses that were brief, usually lasting only 1
to 3 months. The combination of procarbazine with
mechlorethamine, vincristine, and prednisone in the
MOPP regimen, however, resulted in an 81% complete
remission rate in Hodgkin’s disease. Most of these patients
are considered cured. Procarbazine is also used in
various combination chemotherapy protocols for non-
Hodgkin’s lymphomas and small cell anaplastic (oat
cell) carcinoma of the lung. Limited antitumor effects
have been observed against multiple myeloma,
melanoma, and non–oat cell lung cancers. | [Side effects]
The major side effects associated with procarbazine
therapy are nausea and vomiting, leukopenia, and thrombocytopenia.
Skin rashes have been reported, as have
rare cases of allergic interstitial pneumonia. Procarbazine
administration produces a high degree of chromosomal
breakage, and the compound is mutagenic, teratogenic,
and carcinogenic in experimental systems.
Procarbazine may potentiate the effects of tranquilizers
and hypnotics. Hypertensive episodes can result if
procarbazine is administered simultaneously with
adrenomimetic drugs or with tyramine-containing
foods. Rarely, a reaction to alcohol similar to that provoked
by disulfiram may occur. | [Synthesis]
Procarbazine, 1-methyl-2-(n-isopropylcarbamoylbenzyl)-hydrazine (30.6.8),
is synthesized from 1,2-bis-(benzyloxycarbonyl)-1-methylhydrazine, which is alkylated by
the methyl ester of 4-bromomethylbenzoic acid in the presence of sodium hydride, which
forms 1,2-bis(benzyloxycarbonyl)-1-methyl-2-(p-carbomethoxy)benzylhydrazine (30.6.6).
The carbomethoxy group of this molecule is hydrolyzed by sodium hydroxide, and the
resulting carboxyl group is transformed into a acid chloride group, followed by a reaction of
this product with isopropylamine gives 1,2-bis-(binzyloxycarbonyl)-1-methyl-2-(p-iso�propylcarbamoyl)benzylhydrazine (30.6.7). Hydrolyzis of the benzyloxycarbonyl group in
the resulting compound with hydrogen bromide in acetic acid gives the desired procarbazine
(30.6.8). 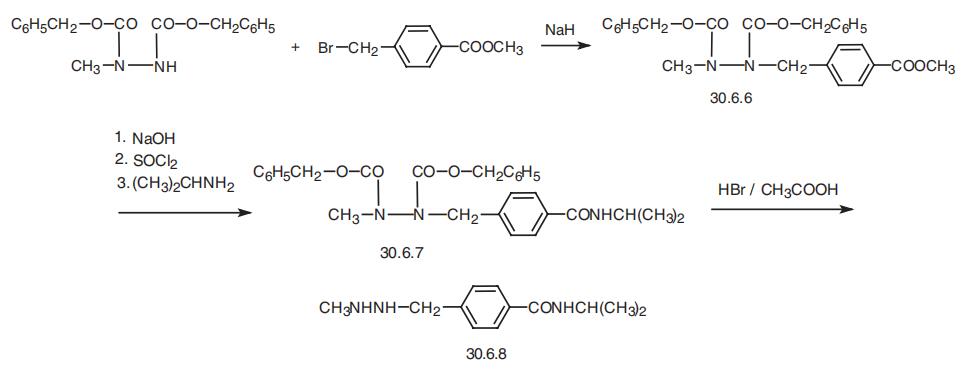
| [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: may produce a disulfiram reaction.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis). | [Carcinogenicity]
Procarbazine and procarbazine hydrochloride are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals. The names “procarbazine” and “procarbazine hydrochloride” are used interchangeably in published studies; because only procarbazine hydrochloride is produced, it has been assumed that procarbazine hydrochloride was the substance under study. | [Metabolism]
Procarbazine is metabolised to an active alkylating agent
by microsomal enzymes in the liver and kidneys and
only about 5% is excreted unchanged in the urine. The
remainder is oxidised to N-isopropylterephthalamic
acid and excreted in the urine, with up to 70% of a dose
recovered in the urine after 24 hours. |
| Safety Data | Back Directory | [Hazardous Substances Data]
671-16-9(Hazardous Substances Data) | [Toxicity]
A substituted hydrazine developed and used as an antineoplastic agent. It
has been found to be carcinogenic in several species, the mechanism being, presumably and by analogy with 1,2-dimethyl hydrazine, the release of the methyl carbonium ion. This compound is
also known to be immunosuppressive and to have adverse effects
on the reproductive system. |
|
|