| Identification | More | [Name]
1,2,4-Benzenetriamine dihydrochloride | [CAS]
615-47-4 | [Synonyms]
1,2,4-BENZENETRIAMINE DIHYDROCHLORIDE
1,2,4-TRIAMINOBENZENE DIHYDROCHLORIDE
benzene-1,2,4-triyltriamine dihydrochloride
1,2,4-Benzenetriamine 2HCL
1,2,4-TRIAMINOBENZENE 2HCL
1,2,4-Benzenetriamine dihydrochloride, pract., 99%
1,2,4-BENZENETRIAMINE DIHYDROCHLORIDE PRACT., 99% (TITR.) | [EINECS(EC#)]
210-428-1 | [Molecular Formula]
C6H11Cl2N3 | [MDL Number]
MFCD00016619 | [Molecular Weight]
196.08 | [MOL File]
615-47-4.mol |
| Questions And Answer | Back Directory | [Uses]
1,2,4-Triaminobenzene Dihydrochloride is a useful reagent for making multicolor fluorescent carbon dots that can be detected via white-?light-?emitting diodes and water. | [Synthesis]
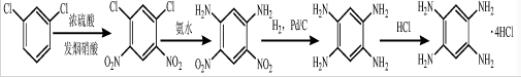
(1) Using m-dichlorobenzene as raw material, dropwise addition at low temperature and high temperature nitration in a mixed acid system of concentrated sulfuric acid and fuming nitric acid to obtain 1,3-dichloro-4,6-dinitrobenzene;
(2) 1 ,3-Dichloro-4,6-dinitrobenzene is placed in a high-pressure reactor in the presence of ammonia water, and the temperature is raised to 145~150 ℃ for ammonolysis reaction to obtain 4,6-dinitro-1,3-benzenediol Amine;
(3) Place 4,6-dinitro-1,3-phenylenediamine, oxygen-free distilled water and palladium-carbon catalyst in a hydrogenation autoclave, and catalyze it under a hydrogen atmosphere of 1-1.5MPa and 85°C After the hydrogenation reaction, the catalyst was removed by hot filtration under nitrogen protection to obtain a 1,2,4-triaminobenzene hydrochloric acid solution;
(4) take the 1,2,4,5-tetraaminobenzene obtained in step (3) The solution was added with concentrated hydrochloric acid, cooled to room temperature, filtered under nitrogen protection, and the filter cake was dried in a vacuum desiccator at 40°C to obtain 1,2,4-triaminobenzene hydrochloric acid.
The molar ratio of m-dichlorobenzene to fuming nitric acid described in the step (1) is 2.2:1, the mass ratio of concentrated sulfuric acid to fuming nitric acid is 3:8~10, and the dropping temperature is -5~0°C, The nitration temperature is 100-104°C.
The reaction time of the ammonolysis described in step (2) is 3.5~4h, and the molar ratio of 1,3-dichloro-4,6-dinitrobenzene to ammonia water is 1:(10~14).
The oxygen-free distilled water described in step (3) is distilled water purified by nitrogen, and the reaction time of catalytic hydrogenation is 3-4h; 4,6-dinitro-1,3-phenylenediamine, palladium-carbon catalyst and oxygen-free The mass ratio of the three distilled water is 1:0.05:10.
The volume ratio of the 1,2,4-triaminobenzene hydrochloric acid solution described in step (4) to concentrated hydrochloric acid is 1:0.2-0.3.
 |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [RTECS ]
DC1953000 | [TSCA ]
Yes | [HS Code ]
29215900 |
| Hazard Information | Back Directory | [Description]
1,2,4-Triaminobenzene hydrochloride is an aromatic triamine with a relatively simple structure. Compared with triaminopyridine, it is easier to synthesize and realize industrialization. To a certain extent, it can replace TAP and 2,5-dihydroxyterephthalene. Formic acid (DHTA) for polymerization. | [Chemical Properties]
PURPLE POWDER |
| Well-known Reagent Company Product Information | Back Directory | [Acros Organics]
1,2,4-Benzenetriamine dihydrochloride, pract., 99%(615-47-4) | [Alfa Aesar]
1,2,4-Triaminobenzene dihydrochloride, 96%(615-47-4) | [Sigma Aldrich]
615-47-4(sigmaaldrich) | [TCI AMERICA]
1,2,4-Triaminobenzene Dihydrochloride,>97.0%(T)(615-47-4) |
|
|